Now, we’ll turn our attention to the basics of the scientific method. We’ll review ideas about observation, replication of results, and other cornerstones of the scientist’s trade.
Scientific Method
Many people define science as any area of learning that applies the scientific method to gain new knowledge. Even without that particular definition of science, the scientific method remains a very powerful tool for learning about the world and universe around us. One reason this method is so powerful is its self-correcting nature.
A key feature of the scientific method is the requirement that all models, hypotheses, and theories must be subject to rigorous testing and the possibility of falsification.
When rigorously applied, these tests weed out wrong ideas. The scientific method can be applied in daily life in areas that we do not normally consider as science. For example, you may hear your car making a funny noise. To diagnose the problem, you, or your mechanic, will first listen to the sound when it occurs. This step corresponds to the first step of the scientific method, which is to observe. When you say it sounds like the thingamabob is going bad, you are essentially formulating a hypothesis. You test the hypothesis when you replace the thingamabob. If the car stops making the sound, the hypothesis passed the test; it was correct. If the car still makes the funny sound, the hypothesis failed the test; it was wrong. You must then formulate a new hypothesis and test it.
The Anatomy of the Scientific Method
As a refresher, remember that the scientific method can be illustrated by a flow chart.
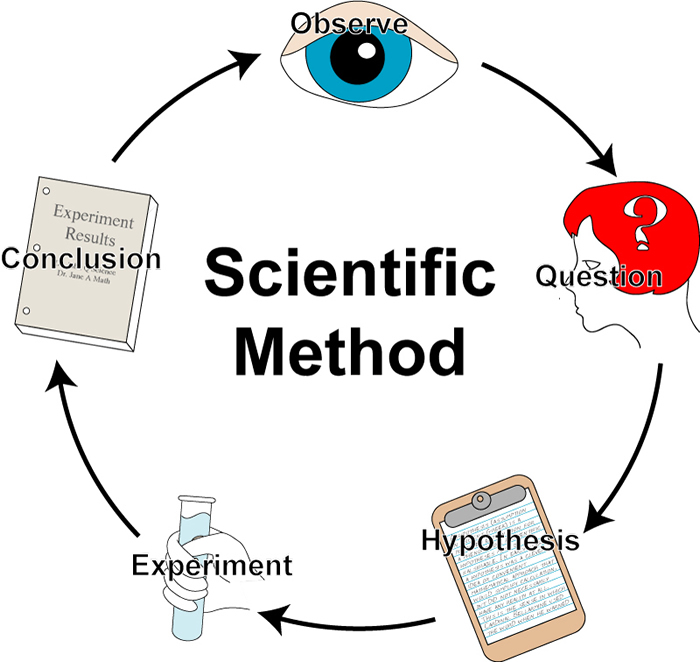
Talk the Talk
The language of science is concise and somewhat esoteric. Click on each of these words if you need to review their meaning.
Observation – any basic watching of something and taking note of behavior, activity, etc. Vision is probably the most often used of the senses, they all can be used for observation. For example, one might observe a frog eating a grasshopper and make a hypothesis that frogs eat insects. Direct observations are the foundation of all natural sciences. Some disciplines like biology have historical milestones as observations by ‘amateurs’ – explained by the fact that there is simple pleasure in observation and learning about our world.
Experimentation – as part of the scientific method, an experiment is any series of organized actions and observations, carried out in a systematic way in order to support or falsify a hypothesis. The experiment is a cornerstone of the empirical approach to epistemology, or knowing the universe.
Inference – is the process of drawing a conclusion based on what one already knows from data or experience. Every night when we go to bed, we subconsciously infer that the sun will rise the next morning (note that sensory perception can also be viewed as an inferential process).
Prediction is a quantitative statement about what will happen in the future under specific conditions.The scientific method is built on testing predictions (based on hypotheses).
Evidence is any data that objectively demonstrate circumstance(s) that indicate or disprove a hypothesis.
Hypothesis is a testable (and therefore falsifiable) extension of a scientific theory that is based on observations.
Theory is a logically self-consistent model that describes the behavior of a natural or social phenomenon and is both originating from, and supported by, observable facts – or data. A theory is a systematic, formalized expression of all previous observations made that is predictive, logical, testable, and has never been falsified.
Law is a scientific generalization based on a huge number of empirical observations and data. Laws of nature are conclusions drawn from scientific experiments that have never been shown to be falsified. A scientific law is a theory that has become indisputable and is often associated with a rigorous quantitative equation that describes it.
Use the Tools
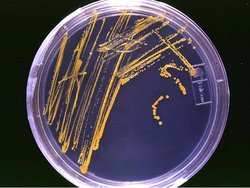 Do you recognize the laboratory tool above? It’s a type of sterile Petri dish called an agar plate, and it’s used to culture bacteria (or fungi). Here’s another quick review of some basic lab equipment.
Do you recognize the laboratory tool above? It’s a type of sterile Petri dish called an agar plate, and it’s used to culture bacteria (or fungi). Here’s another quick review of some basic lab equipment.
- Microscope
- Graduated cylinder
- Balances
- Bunsen burner
- Calorimeter
- Colorimeter
Knowing your way around the metric system is another skill you should have. You should remember the basics: the base units employed by the metric system include meter (length), gram (weight), and liter (capacity). Each of these base-unit terms uses similar prefixes, such as kilo- (one thousand times the base unit) and milli- (one-thousandth of the base unit).
Scientific Experiments
Conducting scientific experiments is an important part of formulating and testing hypotheses in the scientific method. The experiment will test predictions made by the hypothesis or model, so a scientist must be able to plan and conduct valid experiments.
In the ideal case, an experiment will change only one variable at a time. There will also be a control experiment. For example, there is a myth that it is possible to balance an egg on its end on and only on the equinox. A television news show might perpetuate this myth by showing an egg balanced on end during the equinox. However, such an experiment is not properly designed and therefore proves absolutely nothing.
To properly test the hypothesis that there is something special about the equinox that allows an egg to balance on and only on that day, we need a control experiment. The experiment should be repeated on a day that is not the equinox. If the egg balances on that day, then there is nothing special about the equinox, and the experiment proves the hypothesis wrong. It is also important that only one variable be changed at a time. If, for example, you perform this experiment on a sidewalk on the equinox and on the countertop the other day, you are changing two variables. You might reach the incorrect conclusion that there is something special about the equinox when in fact the result stems from the different surfaces.
Complex Sets of Data
In areas of science, such as medical research, where the systems being studied are too complex to change only one variable at a time, the experiment must be repeated on a large number of subjects. A statistical analysis is then needed to tell us if the results are real or simply chance. If a large group of people tries the new treatment, a similar size control group tries a placebo treatment. If significantly more people getting the new treatment are cured, then we can say that the new treatment is effective. We might also use the accepted treatment as a control group to test if the new treatment is more effective than the old treatment. In this type of research, the ideal experiment is a double-blind controlled experiment.
In a double-blind experiment for a medical treatment, neither the experimental subjects (patients) nor the experimenters (doctors) know which subjects are receiving the treatment being tested and which subjects are receiving the placebo. If the patients know which treatment they are getting, psychological effects might affect the cure rates. If the doctors know which treatment a patient is getting, a bias for or against the treatment being tested might affect judgment calls about whether a patient’s condition is improving. So the double-blind experiment is the standard used to judge the validity of these experiments.
Question
You are designing and conducting an experiment to test the claim that magnetic wristbands can reduce the symptoms of arthritis. Which of the experimental procedures listed below is important to make sure your results are valid?
- Making sure that all the patients in the study have magnetic wristbands
- Using a control group of patients who do not have arthritis and also giving them magnetic wristbands
- Limiting the study to fewer than ten patients to make the results simpler to analyze
- Making sure that neither the patients nor the doctors evaluating their symptoms know who has the wristbands that are magnetic
Reveal Answer
The correct choice is D, which describes the double-blind experimental procedure. Choice A does not provide a control group. Choice B provides an incorrect control group. Choice C is too small a sample size to provide accurate statistics.
Experimental Data
Experimental data are often in numerical form. A table of numbers may be sufficient for relatively simple data, but in the case of many or complex data, we often need clearer methods of visualization. It may be difficult to interpret this numerical data if it is simply listed as a table of numbers.
Scientists have devised many ways to visualize their data that depend on the type of data presented. Geologists, for example, frequently use maps. A geological map may show elevations, rock types, fault lines, or many other forms of geologic data. Maps can also be useful in other areas of science. Biologists might use a map to show the habitat of a certain species. Astronomers use maps of the sky to indicate positions of celestial objects.
A biologist might record the percentage of organisms in a sample or in a population that have particular characteristics. Such data might best be expressed as a pie chart.
When scientists want to see if two variables are related, they will plot a graph of these variables. It is customary to plot the independent variable along the x, or horizontal, axis and the dependent variable along the y, or vertical, axis. If the graph looks like a straight line, then we say that the relationship between the two variables is linear. There will then be an equation relating the two variables that is similar to the equation for a straight line in algebra. If the graph does not look like a straight line, then the relationship is nonlinear.
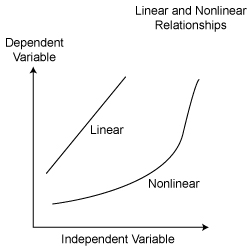
Data Accuracy
There is no such thing as a perfectly accurate measurement. All measurements, scientific or otherwise, will contain some errors. Careful experimental procedure can reduce the amount of error but can never completely eliminate it. Therefore, experimenters must estimate the amount of error in their measurements. A common way to estimate the error in a measurement is to repeat the measurement several times. Then compute the mean (average) and standard deviation of the measurement.
It is important to recognize the difference between accuracy and precision. The two terms sound similar but are not the same thing. Precision refers to the self-consistency of the data. If the standard deviation of repeated measurements is low, then the measurement is precise. It may, however, not be accurate. If you measure the length of your kitchen table with a poorly manufactured meter stick that is not correctly calibrated, all the values will be very close together. The measurement will be precise; however, it will not be accurate. Accuracy refers to how close a measurement is to the actual value. An accurate measurement of the length of your table requires a well-manufactured meter stick that is correctly calibrated.
When the temperature of an object increases, the random motions of the individual atoms and molecules increase; so heat energy is just kinetic energy that is randomized at the molecular level. Energy can also be stored in either electric or magnetic fields, hence light and other forms of electromagnetic radiation are also forms of energy. The chemical energy that is released in a chemical reaction is the potential energy stored by the electrical forces within the atoms and molecules involved in the reaction.
When interpreting experimental data, it is important to look at the error estimates. For example, if the polls for an upcoming election show the Democratic candidate leading the Republican candidate 52% to 48%, does that mean the Democratic candidate is ahead of the Republican candidate? Look at the error estimates. If the error estimate is plus or minus 1%, then the Democrat is winning. If the error estimate is plus or minus 8%, then the race is essentially a dead heat. When deciding if an experimental result agrees with a prediction from the theory, it is essential to also look at the error estimate in the experiment. If the difference between the two numbers is less than the error estimate, the two numbers agree—even if they are not exactly the same number.
Laboratory Safety
When conducting laboratory experiments, you must follow good safety procedures. Failure to follow good safety procedures when working with chemicals can result in severe injury or death. As a teacher, when you or your students perform experiments, make sure that your students follow all the safety rules. You have a legal responsibility to do this. Set a good example by following them yourself. Some things are obvious. For example, when working with a strong acid, don’t drink it or splash it on your skin or eyes. Wear safety goggles and protective clothing in case a strong chemical does splash. Other things are less obvious. Some combinations of chemicals that are reasonably safe by themselves can produce toxic results when mixed. Read the warning labels and the instructions for the experiments. The American Chemical Society has established laboratory safety guidelines to minimize the hazards inherent in laboratory work.
Scientific Pioneers
Like any discipline, science has its shining stars, contributors whose discoveries and experiments changed the way we live and think. Here’s a partial list; be sure to click on the names if you want to review their achievements.
CopernicusIn the sixteenth century, Copernicus suggested that the planets orbit the Sun. Prior to his time, most scientists thought that Earth was the center of the universe and all objects revolved around it. Copernicus displaced Earth from the center of the universe.GalileoIn the early seventeenth century, Galileo was the first person to use a telescope to study the heavens. He made many observations with his telescope that supported the new Copernican model, including the moons of Jupiter, the phases of Venus, sunspots, and craters on the Moon. Most famous for his troubles with the Catholic Church, he was forced to recant his views supporting the Copernican model. After his recantation, Galileo spent the remainder of his life working on the science of motion, called mechanics. Newton later built on this work to develop his laws of motion that provided the physical explanation the Copernican system needed.NewtonIsaac Newton built on the work of Galileo to develop the science of motion, called mechanics. Together with his three laws of motion, Newton’s law of gravity explains the motions we see on Earth and in the heavens. In addition to his work developing mechanics, Newton made important contributions to optics and calculus. He also contributed in several areas outside of science.EinsteinAlbert Einstein’s theory of relativity captured the public imagination in a way that few scientific theories have. In reality, there are two theories of relativity. Einstein’s 1905 special theory of relativity explains the effects that occur when traveling near the speed of light. It also includes the famous equation E = mc2. His 1915 general theory of relativity is an explanation of why gravity occurs. Regarded by many scientists as the crowning intellectual achievement of the twentieth century, general relativity, among other things, predicts the expansion of the universe and the existence of black holes. In addition to his theory of relativity, Einstein also published explanations of the photoelectric effect and the Brownian motion in 1905. The solar-power cells in your solar-powered calculator work on the photoelectric effect. Einstein’s Nobel Prize was for his work on the photoelectric effect, not for his theory of relativity.JustE. E. Just was an African American biologist born in 1883 in Charleston, South Carolina. He overcame the segregation and racism of his era to make fundamental contributions to our understanding of the role that a cell’s nucleus plays in conveying genetic information. In Just’s time, scientists did not yet know that the DNA is contained in the cell nucleus.DarwinIn his book Origin of the Species, Charles Darwin suggested that the natural selection of organisms most fit to survive resulted in biological evolution. Over time, this process resulted in the wide range of species that we see on our planet.LinnaeusBiological organisms can be classified in a number of possible ways. Eighteenth-century Swedish botanist Carolus Linnaeus developed the classification scheme that modern biologists use. The scheme starts with kingdoms as the broadest classification and at each level further subclassifies organisms down to the species level.MendelGregor Mendel was an Austrian monk who performed experiments with breeding pea plants in the nineteenth century. Mendel’s experiments laid the foundation for our modern understanding of genetics.JulianPercy Lavon Julian was born in Alabama in 1899. Despite the segregation and poor schools of his era, he made important contributions to science. During the middle of the twentieth century, his research led to the development of low-cost cortisone for the treatment of arthritis and physostigmine for the treatment of glaucoma. He also made synthetic male and female reproductive hormones and a foam used for putting out oil and gas fires.CarsonThe 1962 publication of Rachel Carson’s classic book, Silent Spring, was an important influence on the environmental movement. Carson outlined the effect that the pesticide DDT had on birds. Ten years later, its use was banned, and bird populations began to recover. She helped people understand the unintended effects that chemicals can have on the environment.MuirJohn Muir lived in the late 1800s and the early 1900s. A naturalist who was among the first to recognize how different species were interconnected, Muir was an early advocate of the importance of understanding and preserving ecology. He was also instrumental in founding Yosemite national park, and the national park and wilderness system in general.MendeleevDimitri Mendeleev was born in Siberia in the nineteenth century. When he first started teaching chemistry, he found no suitable textbooks to use, so he wrote one. For this book, written between 1868 and 1870, Mendeleev organized the known elements in order of their atomic weights. We now use atomic numbers, but they were unknown in his time. Mendeleev noticed that periodic similarities in the chemical properties of the elements in his table occurred if he left certain places blank. He was bold enough to predict that elements would be discovered to fill in these blanks. He was right. The chart he developed became the periodic table of the elements and provides much of the basis for our understanding of modern chemistry.DaltonJohn Dalton was a British teacher who in 1808 proposed that matter was made up of atoms. His realization came from his study of the meteorological problem of water vapor in the air. We now know that not all aspects of Dalton’s atomic hypothesis are correct, but the major idea that matter is made up of individual atoms is correct.BohrNiels Bohr proposed his model of the atom in the early twentieth century. Bohr suggested that the electrons orbit the nucleus in very specific allowed energy levels. When electrons jump between levels, spectral lines are produced. Bohr also made many other important contributions to quantum mechanics.CurieMarie and Pierre Curie were a husband-and-wife-team who did much of the early work in understanding radiation. They first isolated the element radium and then isolated other radioactive elements. They pioneered the use of radiation to treat cancer.PasteurBefore Louis Pasteur’s time, disease was rampant in large part because people did not understand its causes. Pasteur pioneered germ theory and the process used to kill germs found in milk. After people understood that germs caused diseases, they were able to reduce the spread of disease by improving sanitation. He also developed a cure for rabies and made other contributions to science.
Review
- The scientific method is based on observation and replicating results.
- The metric system was developed in France in the late eighteenth century. Its base units include the gram, the meter, and the liter.
- Good scientific experiments change one variable at a time.
- A control experiment (or group) is one in which the variable being tested does not change; this provides the scientist with a way to see the effects of a changing variable in other groups.
- In double-blind experiments, neither the researchers nor the subjects tested know who is in the control group and who is not. (These experiments radically reduce bias.)
 username@email.com
username@email.com


