 username@email.com
username@email.com
In this lesson, we will review the properties of matter, the organization of elements on the periodic table, and the types of chemical bonds. We’ll also review chemical reactions and the properties of chemical solutions.
Chemistry is the branch of science that focuses on the composition of matter—the material substances that make up the universe. The basic definition of matter is anything that has mass and takes up space. In short, matter is what the universe is made up of and what we can categorize it into.
The universe is composed of roughly 109 elements. The basic unit of an element is an atom. Every atom of each element has the same properties and differs from the atoms of all other elements. A compound is formed when two or more atoms combine in a fixed proportion, and most substances in our universe have formed compounds.
If atoms form a unit with a specific number of atoms of two or more elements, the combination is called a molecule. A molecule is the basic unit of a compound, like water. Some elements also tend to exist as molecules of two or more atoms. Many elements are in their most stable form when they bond with themselves, forming diatomic molecules. Nitrogen and oxygen, two of the most common gases in our atmosphere, are examples of diatomic molecules (N2 and O2).
Matter that has a uniform composition is called a substance. The key to identifying a substance is that its composition and properties are always the same. Pure water is a substance; apple juice is not. Two samples of apple juice might look and taste very different, depending on the type of apples, the length of time between picking them and pressing for juice, and many other variables.
There are two kinds of properties that you can use to tell one substance from another—physical properties and chemical properties. A physical property can be determined without changing the substance into something else, whereas chemical properties are the substance’s ability to combine with other substances and can only be measured by changing the substance.
Some of the properties of matter depend on the amount of matter present. These extensive properties (or extrinsic properties) include its mass and its volume. Other properties, known as intensive properties (or intrinsic properties) of a substance are independent of the amount of material present. Intensive properties include density, melting point, boiling point, and electrical conductivity.
Most materials that you use are not pure. Although water is a substance, your tap water is not pure. In addition to water, it contains dissolved minerals — tiny amounts of metals, carbon dioxide, and oxygen from the air. Like apple juice, tap water is a mixture — a combination of two or more substances that retain their individual properties. Homogeneous mixtures are made of two or more distinct substances, but they have a constant composition within a sample. Clear apple juice is an example of a homogeneous mixture.
Homogeneous aqueous mixtures are also known as solutions. The term solution usually refers to a mixture in the liquid state, although homogeneous mixtures of gases or solids can also be called solutions. Heterogeneous mixtures consist of substances that retain their distinct characteristics and do not blend completely. Orange juice with pulp is an example of a heterogeneous mixture. Although a compound is made of more than one element, a compound is not a mixture. In a compound, atoms are held together in a fixed proportion, making a single chemical substance. In a mixture, two different substances are mixed together, but the proportion can change.
Which of the following is a homogeneous mixture?
Choice C is the correct answer. Aluminum foil is a single, uniform substance and the other choices are heterogeneous mixtures.
The first subatomic particle to be discovered was the electron. Electrons have a negative electric charge and a negligible mass. Not long after the electron was discovered, the proton was also discovered. The proton has a positive charge and is about 1,840 times more massive than an electron. If an atom has equal numbers of protons and electrons (necessary to make it neutral), almost all of its mass consists of protons.
After more observations, scientists realized that protons and electrons did not account for all of the mass of an atom. The rest of the mass was made up of a particle called the neutron, an electrically neutral part of each atom’s nucleus. All of the protons and neutrons are clustered together in the center of an atom. This cluster, known as the nucleus, contains almost all of the mass—and very little of the volume—of an atom. The discovery of protons and neutrons, along with the determination that they all cluster together in the center of the atom, led to a model of the atom that looked something like this illustration.
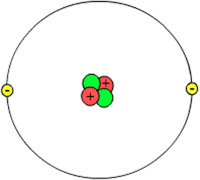
The number of protons in the atomic nucleus is called the atomic number of the element. The atomic number of helium is 2. The mass of an atom is located almost entirely in its nucleus. The mass number of an atom is the sum of the number of protons and the number of neutrons. Most carbon atoms have six neutrons and all carbon atoms have six protons, so most carbon atoms have a mass number of 12.
How many neutrons does an atom of zinc-64, 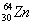 , have?
, have?
The correct choice is B. The number of neutrons is the mass number, 64, minus the atomic number, 30.
There are a variety of different chemical and physical properties of the elements, but they are patterns of similarity among different elements as well. The periodic table is a method for organizing the elements based on these similarities.
The periodic table is an important tool for all scientific endeavors. Understanding the significance of Groups IA through VIIIA is the key to the rest of the periodic table. The increasing Group A number corresponds to the increasing number of valence (outer) electrons. Group VIIIA elements all (except He) have 8 valence electrons, hence the rule of octet. Helium (He) is such a small atom that only two electrons have room to fit so near the nucleus and fill the outer shell. The elements in Groups IA and IIA easily lose electrons to become positive ions. The tendency to lose electrons easily identifies elements that are commonly called metals. Group IIIA elements tend to lose electrons but not as readily as the Group IA and IIA elements. They often combine with other atoms by sharing electrons. The elements in Group VIA and VIIA easily gain electrons to become negative ions. The tendency to gain electrons easily identifies elements that are commonly called non-metals. The Group V elements tend to gain electrons, but not as readily as Groups VIA and VIIA. They too often combine with other atoms by sharing electrons. The Group IVA elements invariably combine with other atoms by sharing electrons, they do not readily transfer electrons. The Group B elements have more complicated electron structures and will be discussed shortly.
Elements in each column of the periodic table exhibit similar properties and are said to be in the same elemental group or family. For example, sodium and potassium, both in the first column of the table, have very similar chemical and physical properties because they share the same number of electrons in their outermost energy level. They are in Group IA which means they each have one electron in their valence shell.
The periodic table is also arranged by increasing atomic number (number of protons) from left to right in each row. Many elements can be classified by their position on the periodic table. Some of the regions of interest are:
Metals are the elements on the left and center of the table and the lower section of the right side of the table. Metals tend to be hard, shiny elements that conduct heat and electricity well.
Nonmetals are the elements on the right side of the table. Nonmetals form soft, brittle solids. Many of them are gases at room temperature. Nonmetallic elements do not conduct heat or electricity well.
Metalloids are the elements in a zigzag line that separates metals and nonmetals. These elements share some properties of both metals and nonmetals.
Atoms react with one another in different ways, forming three basic types of chemical bonds:
Ionic bonds generally form between metal and nonmetal atoms, but always with both positive and negative partners.
Covalent bonds generally form between nonmetal atoms.
Metallic bonds form between metal atoms.
When forming an ionic bond, the metal atom transfers its valence electrons to one or more nonmetal atoms. An atom with an unbalanced number of positive and negative charges is called an ion. The metal atom, in this case, becomes a positively charged ion called a cation and the nonmetal atom becomes a negatively charged anion. The cation and the anion both have more stable electron arrangements. Because they have opposite charges, the ions are attracted to one another, forming a strong bond.
In nonmetal atoms, the valence electrons generally are not removed to form a cation, so a different type of bonding occurs in nonmetals. In a covalent bond, two atoms share a pair of electrons. Usually, each atom contributes one electron of the pair, although in some cases, one atom can contribute both electrons. This type of chemical bonding is illustrated below.

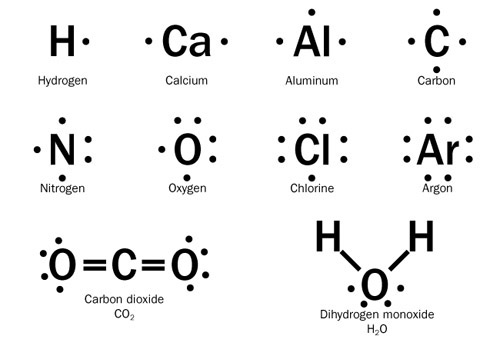
Chemical bonds can also be depicted using Lewis dot diagrams. These are drawings that incorporate the symbol for the element, surrounded by dots that represent valence electrons and lines that represent bonds. This illustration shows the Lewis dot symbols for several elements and compounds. The chemical bond can be indicated by a line, as shown here, or by two dots side by side. Notice that in carbon dioxide, each oxygen atom is bound to the carbon atom by a double bond, two pairs of shared electrons.
On Earth, all matter occurs in one or more of three phases: solid, liquid, or gas. There are other phases of matter, but they are unusual under normal conditions. Under any given set of conditions, the phase of a substance depends on the interaction of its particles—atoms, molecules, or ions.
The phase of a particular substance is a function of temperature and the forces between its particles. At room temperature, substances that experience weak interactions are gases. For example, the noble gases are electrically very stable and have very low melting points. The molecules of water, on the other hand, have a larger degree of polarity. Shared electrons tend to spend more time around the oxygen atom than around the hydrogen atom. One part of the molecule is somewhat negative and the other part somewhat positive. Because of this, water molecules are more strongly attracted to one another than are the noble gas atoms, and water is liquid at room temperature. The strong electrical attraction of the ions in ionic compounds, such as sodium chloride, means that they are solid at room temperature.
Temperature is a measure of the motion, or energy, of particles. As temperature increases, particles move faster. Eventually, the particles of a solid, such as ice, move fast enough to overcome some of the attractive forces and the ice melts to form liquid water. As additional energy is added, the particles move even faster, becoming a gas. There is a second factor in determining the phase of a material: As pressure increases, the particles are forced closer together.
Matter is constantly changing. The food that you eat becomes part of your body; gasoline becomes carbon dioxide, water, heat, and the energy that runs your car; the ink in your printer cartridge binds with paper to form a document. The process by which atoms of substances rearrange to form new substances is called a chemical reaction. Some indications of a chemical reaction include a change in temperature, such as in fire, a color change that indicates the presence of a different substance, and the generation of electrical current by a battery. Chemical reactions are written using shorthand similar to a mathematical equation:
reactant A + reactant B → product C + product D
But there are many different types of reactions. For example, burning hydrogen in oxygen can be written as:
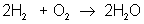
You can see that the equation for the reaction of hydrogen and oxygen indicates more than just the reactants and products. It further shows that two molecules of hydrogen react with a single molecule of oxygen to produce two molecules of water. The total number of hydrogen atoms (four) and the total number of oxygen atoms (two) are the same on both sides (reactants and products) of the reaction. Unlike nuclear reactions, chemical reactions do not change the identity of any atom. There are always the same numbers and types of atoms before and after the reaction; they are just arranged differently. Although new combinations of molecules exist, the equation must be balanced to show all atoms are accounted for.
Equations are balanced by changing the number of molecules in the equation. Coefficients, which indicate number of units, can be changed. Subscripts that identify the number of atoms within a molecule, however, cannot be changed.
In order to balance an equation, we start with a basic equation that shows all the reactants and the products. For example:
ethane + oxygen → carbon dioxide + water
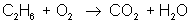
reactant: C – 2 ; H – 6 ; O – 2
product: C – 1 ; H – 2 ; O – 3
Adjust coefficients so that carbon is balanced on both sides:
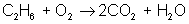
reactant: C – 2 ; H – 6 ; O – 2
product: C – 2 ; H – 2 ; O – 5
Next, adjust coefficients so that hydrogen is balanced on both sides:
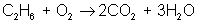
reactant: C – 2 ; H – 6 ; O – 3
product: C – 2 ; H – 6 ; O – 7
Then adjust coefficients so that oxygen is balanced on both sides:
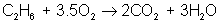
reactant: C – 2 ; H – 6 ; O – 7
product: C – 2 ; H – 6 ; O – 7
And finally, make all coefficients whole numbers:
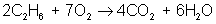
There are millions of different chemical reactions that occur every day all around us. Chemists organize information about reactions by classifying them by patterns of chemical change. This classification helps predict the products and conditions of a chemical change. Most chemical reactions fall into four categories:
A synthesis reaction is a reaction in which two or more reactants combine to form a single product (also called a composition reaction):
A + B → C
An example of a synthesis reaction is:
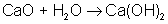
AB → A + B
An example of decomposition is the breakdown of hydrogen peroxide when it is exposed to light:
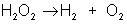
AB + C → A + BC
An example of a single-displacement reaction is:
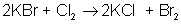
AB + CD → AC + BD
An example of a double-displacement reaction is:
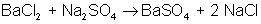
Which of the following is a synthesis reaction?
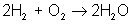
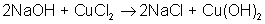
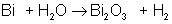
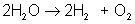
The correct choice is A. B is a double-displacement reaction; C is a single-displacement reaction; D is a decomposition reaction.

There are many different compounds that form acidic or basic solutions. Common acids include foods, such as orange juice and vinegar, as well as acids that are dangerous to handle, such as the solution in automobile batteries. Common bases include antacid tablets that settle your stomach and drain cleaner that can dissolve clogs in sewer pipes.
General properties of acids include:
General properties of bases include:
Notice that the final property listed for acids and bases is that they react with one another to form a salt and water. This process is known as neutralization.
Acids and bases vary in strength. Very strong acids, such as concentrated sulfuric acid in batteries, and very strong bases, such as the sodium hydroxide in drain cleaner, are dangerous substances. Both strong bases and concentrated acids dissociate violently in water with considerable spattering and releasing of large quantities of heat. They also react strongly with body tissues and can cause severe “burns” and even death. On the other hand, weak acids, such as water, and weak bases, such as blood, are essential for life.
The relative strength of acids and bases is measured on a scale known as the pH scale. The pH is the negative logarithm of the hydrogen ion concentration. That means the pH of an acidic solution, with a hydrogen ion concentration of 1 × 10-1 M is equal to 1. The pH of a basic solution with a hydrogen ion concentration of 1 × 10-13 M is equal to 13. Solutions with very low pH values, such as 1 or 2 are considered strong acids, while solutions with a pH value of 5 or 6 are weak acids. Strong bases have high pH values, such as 13 or 14, while weak bases are solutions whose pH is in the 8 to 9 range.
An aqueous solution has a pH value of 6. This solution is classified as a
The correct answer is B. A pH value of 6 indicates a weak acid.