 username@email.com
username@email.com
We will now cover the biology content in more depth; starting with the building blocks of life, organic molecule. We will review the types of organic molecules, what they are composed of, and how they function in living organisms. The biochemical basis of life is fundamental to all other aspects of biology and the life sciences.
We have just covered the most important aspects of Physics in depth. The last lessons covered electromagnetism and optics, the properties of waves, light, and the electromagnetic spectrum.
As we understand the universe, all matter is fundamentally composed of atoms. Depending upon the numbers of protons, neutrons, and electrons, an atom can be any one of at least 259 known elements (with perhaps half a dozen more yet to be discovered). Of these 259 elements, only about a dozen are important for life — and the molecules formed by these elements are called organic molecules. Organic chemistry is one of the most important and complex disciplines within chemistry and overlaps considerably with biochemistry and biology. Living things are composed primarily of organic molecules.
Organic molecules are those that have a carbon skeleton with hydrogen and/or other functional group attached. Because carbon has four valence electrons, it tends to form covalent bonds with up to four other atoms. Coincidentally, the energy used by living things is usually stored in the covalent bonds of organic compounds.
Hydrocarbons or alkyls are the simplest organic compounds, containing only carbon and hydrogen. Alkanes are saturated hydrocarbons, meaning there are no carbon double bonds present. Methane, shown below, is an example of an alkane. Alkenes, on the other hand, are characterized by having a double bond; they are unsaturated hydrocarbons. The degree of saturation of a hydrocarbon influences its reactivity and shape. Below is also shown an example of a simple alkene, ethene.
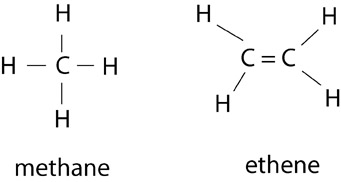
Examples of Alkanes
The functional groups attached to carbon compounds determine the reactivity and classification of more complex organic molecules. Classes of organic compounds are often represented by the functional group bonded to a generic organic group that can vary among the amino acids. These generic organic groups are sometimes represented as an R. Likewise, there are other symbols for specific types of functional groups.
| Methyl Group | |
|
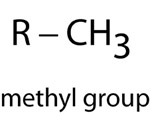 |
| Ether Group | |
|
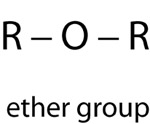 |
| Hydroxyl Group | |
|
 |
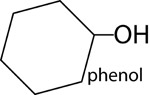
| Phenols | |
|
 |
| Sulfhydryl Group | |
|
 |
| Carbonyl Group | |
|
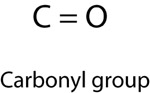 |
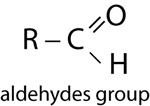
| Aldehydes | |
|
 |
| Ketones | |
|
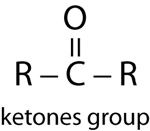 |
| Carboxyl Group | |
|
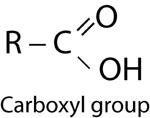 |
| Esters | |
|
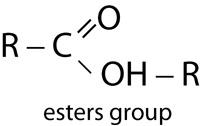 |
| Amine Group | |
|
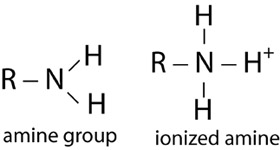 |
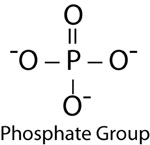
| Phosphate Group | |
|
 |
Complex organic macromolecules called polymers are formed from simple organic monomers. Living things are composed primarily of only four kinds of organic polymers: carbohydrates, lipids, proteins, and nucleic acids. Each type of these macromolecules is made of a distinctive combination of organic monomers. Organic monomers undergo dehydration synthesis (also called condensation reaction) to produce complex polymers. In a condensation reaction, one monomer loses an H+ ion and the other loses an OH–; to create water (H2O) and a new bond linking the formerly two molecules into one. In the opposite reaction, called hydrolysis, polymers are broken down into monomer subunits by the addition of water.

Common dietary sources of carbohydrates
Carbohydrates are standard energy storing molecules and are commonly called sugars and starches. The monomers of carbohydrates are called monosaccharides or sugars. They produce polymers called polysaccharides or starches by forming glycosidic bonds. There are several classes of carbohydrates that we will discuss in detail later.
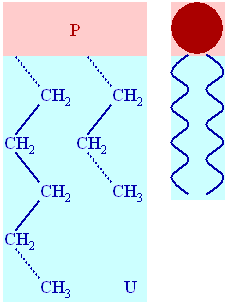
Example of lipid structures
Lipids typically contain long fatty acid chains, are non-polar, and water insoluble. Fats, like triglycerides and oils, are lipids composed of glycerol bonded to fatty acid chains. Other lipids, such as steroids, contain distinctive ring structures and can have powerful biological properties.
Proteins have several levels of structure, but all proteins are composed of amino acid monomers joined by peptide bonds in dehydration synthesis reactions. Proteins are very important in controlling metabolism in their action as enzymes.
The monomers of the nucleic acids DNA and RNA are called nucleotides. Nucleotides form phosphodiester bonds in dehydration synthesis reactions to form the DNA backbone. Another important nucleotide in all living things is called adenosine triphosphate (ATP).
ATP is a very important molecule in living things. It is often called the energy currency of the cell. It is composed of a pentose sugar called ribose, an amine called adenine, and three phosphate groups. The line of phosphates contains very high-energy bonds that are easily broken. Energy is transferred by dehydration synthesis reactions by making and breaking high-energy bonds in ATP.
Examples of dehydration synthesis reactions for three of the four main classes of organic macromolecules are shown below.
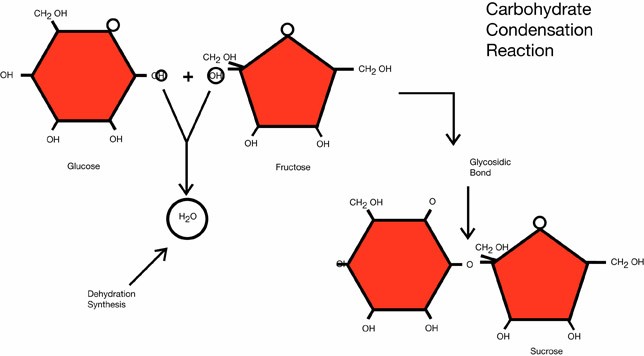
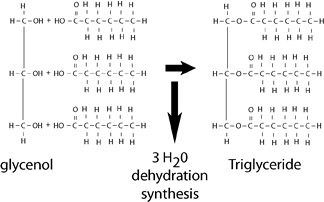
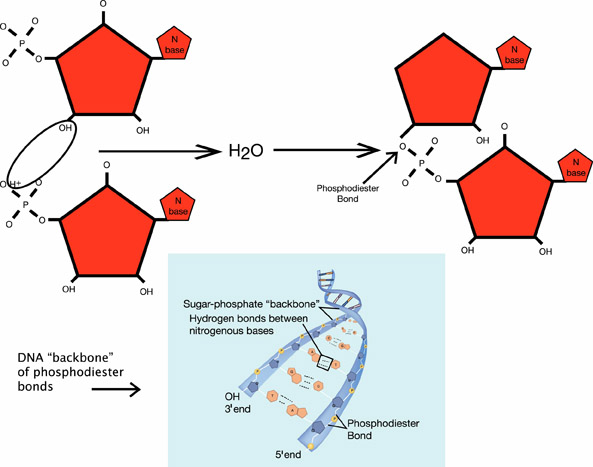
Dehydration synthesis in a nucleic acid
Hydrolysis reactions for each type of macromolecule are the opposite process of that shown above. In hydrolysis, water is split by hydrolytic enzymes, breaking it into an H atom and an OH group. These react to form simple monomers when the molecular bonds of the polymer are broken.
What is formed by the dehydration synthesis of sucrose?
The correct answer is C. Dehydration synthesis builds complex molecules from simple ones, and water is produced as a by-product.
In this lesson, we will review the kinds and properties of carbohydrates.
Carbohydrates are important sources of quick energy. Energy used by cells is released from carbohydrate bonds such as glucose. Carbohydrates also function in energy storage as starch and glycogen, and as important structural elements, particularly for plants, fungi, and the cell walls of arthropods.
The simplest carbohydrates, monosaccharides, are classified by the number of carbons present in the chain. For example, a triose contains three carbons; a pentose contains five, and a hexose, such as glucose, contains six carbons. Sugars may also be classified based on functional groups present. An aldose contains an aldehyde group; a ketose contains a ketone group. Glucose is an aldohexose; it is a six carbon sugar with an aldehyde attached. Like many other systems of classification, the naming scheme can be more general or more specific, so many macromolecules can have more than one name. For example, glucose is also one of the many types of hexose.
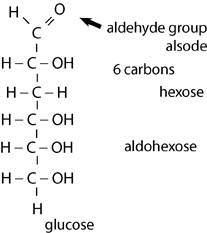
Glucose
Monosaccharides often exist as isomers. Isomers are molecules with the same chemical formula and often the same kinds of bonds between atoms, but in which the atoms are arranged differently. Glucose, for example, can have as many as sixteen different configurations of atoms. This can seem unimportant, but is actually crucial for most biomolecules. Various isomers of glucose are named according to location of functional groups. Unique isomers of simple carbohydrates are involved in many important metabolic pathways.
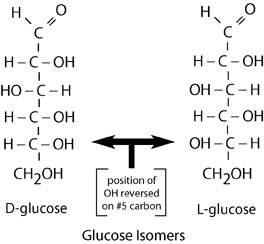
Example of the isomers of glucose
Monosaccharides may also exist as straight chains, bent structures, or as ring forms. When there are five or more carbons in a chain, very stable ring structures called hemiacetals may form. This occurs when the carbon on one end of a chain reacts with the hydroxyl on the opposite end. Several combinations are possible in pentose and hexose sugars. In living things, most five and six carbon monosaccharides most often occur in ring forms. This is primarily due to the high stability of these molecules relative to their sister isomers.
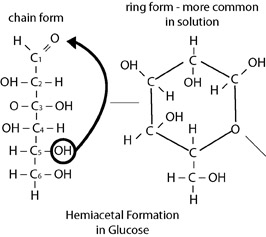
Example of ring structure of hemiacetals
An oligosaccharide is a more complex carbohydrate composed of two to ten monosaccharide subunits. A disaccharide is an oligosaccharide composed of two monosaccharides, such as the sucrose molecule shown in the dehydration reaction for carbohydrates. Polysaccharides are very complex, composed of more than 10 monosaccharides. Cellulose, starch, glycogen, and chitin are all biologically important polysaccharides.
Cellulose is the most abundant polymer on Earth. Cellulose is the primary structural component of plant cell walls. So, if you are wearing cotton, sitting on a wooden chair, or taking notes, you are wearing, sitting, or writing on cellulose. Moreover, almost all of our dietary fiber comes in the form of cellulose. Cellulose monomers are linked together through 1-4 glycosidic bonds in a straight chain. In cellulose microfibrils, hydroxide groups in the monomers hydrogen-bond with each other, holding the chains firmly together creating a lattice structure. This gives cellulose fibers high tensile strength. In plant cell walls, cellulose forms a matrix with hemicellulose and lignin to give plants support.
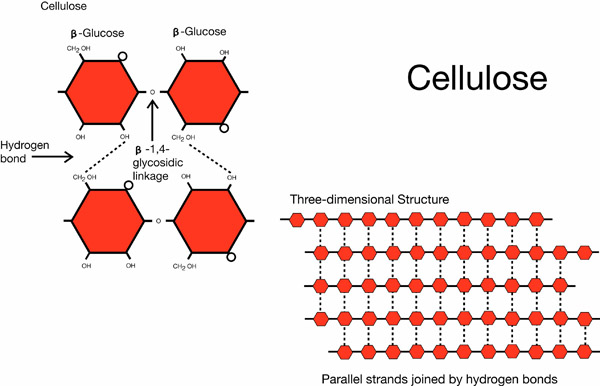
Cellulose
Starch and glycogen are energy storage carbohydrates. Starch is the primary form of energy storage in plants, while glycogen is the form of carbohydrate energy storage in animals. Starch and glycogen are polymers of alpha glucose. Amylose, a form of starch, has alpha 1-4 glycosidic bonds; while the starch amylopectin and glycogen have alpha 1-4 glycosidic bonds and alpha 1-6 glycosidic bonds. Starch polymers occur as either unbranched or branched helices, while glycogen occurs as complex branched helices.
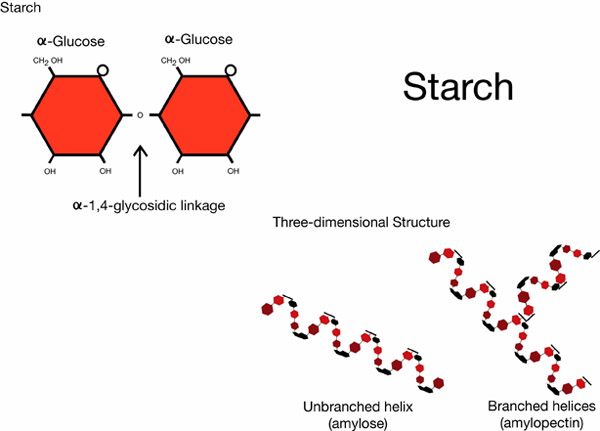
Example of a starch
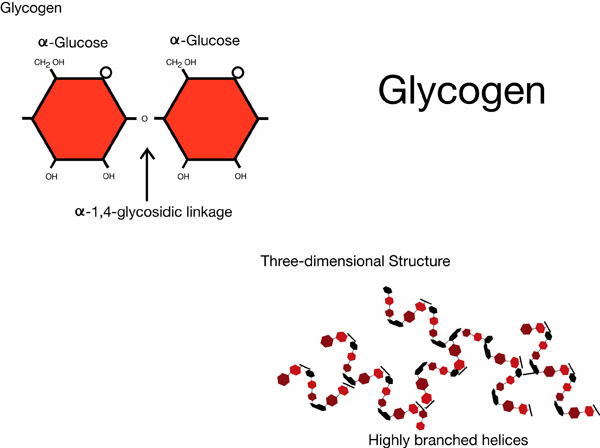
Example of glycogen
Chitin is a complex carbohydrate common to fungi and arthropods. It is a primary component of fungal cell walls and of the hard exoskeleton of insects and crustaceans. Chitin is structurally similar to cellulose. It is made out of monomers of acetylglucosamine linked together in the same way as glucose units in cellulose. Chitin differs from cellulose in that one hydroxyl group on each monomer is replaced by an acetylamino group. This allows for increased hydrogen bonding between adjacent polymers and increased strength.
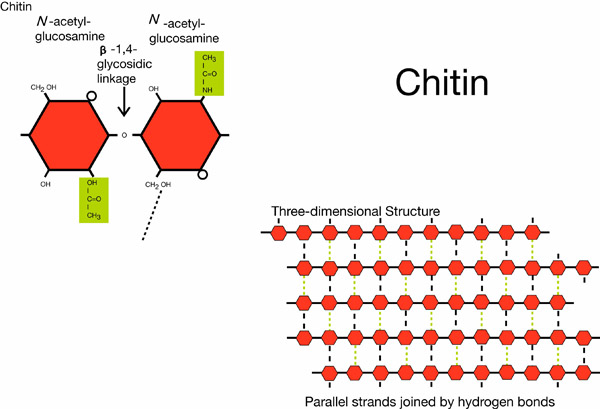
Chemical structure of chitin