username@email.com
username@email.com
Now we will turn to the atomic structure, an atom’s stability, and one of the best ways to show electron distribution, with a Lewis dot structural diagram.
We have reviewed the essential nature of electron orbits and their role in determining many of the fundamental differences between the classes and groups of elements and how they interact.
Atoms tend to arrange themselves in a way that is most stable, or at the lowest energy. The periodic table is arranged in such a way to help us predict reactions based on atomic structure and the shared characteristics of groups of elements that share a set number of valence electrons. Most elements don’t exist in their pure forms as they are depicted on the table because they are more stable when reacted with other elements and substances. An interesting exception can be found in group 8A containing helium, neon, argon and the other noble gases. These elements at the end of each row are so stable that it is difficult to make them react under any conditions. These elements have full electron configurations in their outermost energy level orbits (rule of octet again), and hence no potential for picking up or sharing electrons belonging to other elements. For this reason, they are called inert and are most stable in their pure elemental forms.
Outside of these noble gases, different elemental groups vary in how stable they are in their pure forms, or how easily they form compounds with other elements. On the other end of the spectrum, for example, are the alkali metals and the halogens, two groups that are extremely reactive, almost never existing in their pure elemental forms in nature. These two groups have an electron to donate or have room to gain one electron from another atom. For this reason, these two groups are often found with each other as ionic substances and with most other elements.
Atoms have three basic types of subatomic particles: electrons, protons, and neutrons.
The nucleus is the very dense and positively charged center of the atom. The electrons are negatively charged, existing in clouds about the nucleus to balance the atom. For an atom to bond to another atom, it must somehow share or transfer electrons with or to a neighboring atom. Electrons are the keys to reactivity between elements, primarily because they are negatively charged and are attracted to the positively charged nuclei of the two atoms. Some bonding is called ionic because the electrons physically move from one atom leaving behind a positive ion, to another atom creating a negative ion. The two oppositely charged ions then come together to form ionic substances. Covalent bonding occurs when neither atom gains or loses electrons, but rather are shared equally by both atoms. The electrons are shared by the atoms involved in the reaction. Ionic bonds require large amounts of energy to break as solids due to their stable lattice structure, although most ionic substances easily dissolve in water with electrons remaining with the non-metallic atom.
Let’s look at some simple examples with familiar substances in order to understand bonding and compounds better. If you examine the electron configuration of hydrogen (element number 1 & atomic number 1), you will find it to be the simplest of atoms with one s orbital containing one electron. For hydrogen to be most stable, it must find another electron to have the configuration similar to helium. Helium has two protons and two electrons equally balancing a positive and negative charge. But hydrogen has only one proton so it doesn’t have the potential to hold two electrons like helium does. So if another atom of hydrogen happens to come by with its lone electron, the two atoms can share the pair of electrons making them both more stable. The resulting compound H2 has an equal attraction for the pair and thus is said to “share” electrons. Chemists label this type of bond as covalent, meaning they have an equal valence or attraction for the negative charge. Hydrogen is an excellent example of an element that forms diatomic molecules with itself in order to reach a more stable electron distribution. Try to think of other elements that do this readily (hint — almost our entire atmosphere is comprised of diatomic molecules).
Hydrogen can also bond to oxygen (element number 6) and will form the substance water, formula H2O. Oxygen has two energy levels. The innermost level has the same configuration as helium, which has great stability. The outermost layer is lacking two electrons to have the stability that neon has with a full outer shell. So with the need for two electrons, each of two hydrogen atoms can share its one electron to make a stable configuration with oxygen, and at the same time make a stable configuration for itself similar to helium. Because oxygen has 6 protons (six times more positive charge than hydrogen) the shared pair of electrons is pulled a little closer to the oxygen than the hydrogen, which has one proton. While this is still considered to be a covalent bond, it is called polar covalent because the electrons are not equally attracted to both of the atoms in the bond.
Hydrogen can also bond to chlorine, element number 17. Chlorine has 17 protons and 17 electrons. The first energy level has a stable configuration similar to helium and is very stable. The second energy level has a stable configuration similar to neon and is also very stable. But the third energy level needs only one electron to have the stability of argon (one of the noble gases). Hydrogen can bring its single electron to the partnership, but when this bond occurs, the pull on the electrons is very strong towards the chlorine atom. In fact, it is so strong that in water, the two atoms will separate and the electrons will go with the chlorine atom to form an ion. An ion is simply an atom with a charge, or a different number of electrons than protons. In this case, chlorine has 17 protons, but 18 electrons; thus it is negatively charged. It is now called a chloride ion, (Cl–). But it is more stable than it was uncharged. The hydrogen that has now lost its electron is also an ion but it is positively charged (H+). That is why this type of bonding is labeled ionic because it often forms ions when the bond is broken. This substance, HCl, is responsible for the activity that occurs in your stomach (also known as stomach acid) and is one of the most important biological acids, or H+ donors.
So an element can form any kind of bond, depending on what other elements are interested in the same electrons in the vicinity. All elements can shift electrons to gain stability and the most stable configurations are those with a full outer shell similar to the noble gases in group 8A.
An American Chemist, Gilbert Newton Lewis, devised a simple way to demonstrate the octet rule using what are known today as electron dot structures. The dots come from the number of electrons that exist in the outermost shell.

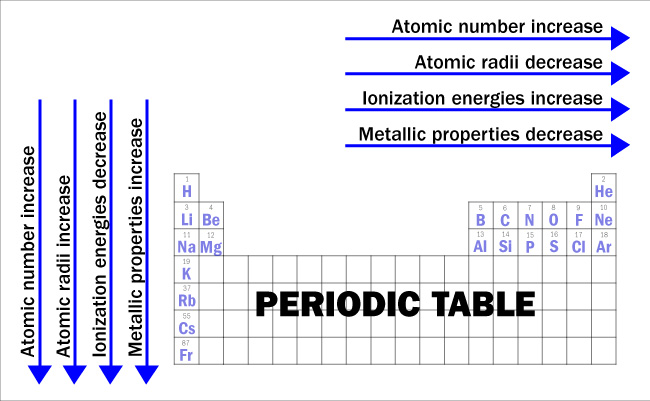
Based on the atomic structures and the arrangement of the elements on the table, there are four trends that explain the basis for many of the potential reactions that can occur between the elements. First, atomic number increases from top to bottom and from left to right, which means that there is a greater positive charge due to increasing numbers of protons. Second, atomic radii decrease from left to right because the greater increase in the positive nuclear charge for the same energy level has a tendency to pull electrons (negatively charged) closer and thus make the clouds a little more dense (less volume). Atomic radii increase from top to bottom because of the greater distance from the nucleus, and the increasing number of energy levels. The third trend is that ionization energies increase from left to right because it becomes harder to remove electrons (negatively charged) that are held more tightly by the greater positive charge in the nucleus for the same energy level. Ionization energies decrease from top to bottom because the distance from the nucleus and the increasing number of energy levels makes it easier to remove the outermost electron. Finally, metallic properties decrease from left to right and increase from top to bottom. Metallic properties depend on the ease with which an electron can be removed from an atom. Metals have electrons that are more loosely held in the outer shells and the freedom of movement is what accounts for the conduction of electricity and heat and other properties.