 username@email.com
username@email.com
We are now going to review the electron orbits, their shapes, and distribution on the periodic table. This will set the stage for understanding the different kinds of reactions and bonds that occur among elements, molecules, and compounds.
In the previous lesson, we reviewed the periodic table, its history and development. We know that Mendeleyev, the great Russian chemist, was an excellent predictive scientist who was able to foresee the discovery of dozens of elements before they were formally recognized. We also reviewed the nine main classes of elements (alkali metals, alkaline Earth metals, the rare Earths, transition metals, post-transition metals, metalloids, the non-metals, halogens, and noble gases), the 18 groups of elements, and their constituent properties.
Quantum mechanics describes the probability for finding an electron within the specific dimensional spaces described by Schrödinger’s wave equations. The area in which there is a probability for finding an electron is called the orbital space. The orbital space-shapes, called orbital configurations, are described by mathematical equations which predict the possibility for finding an electron in that space. Only two electrons can ever occupy any orbital.
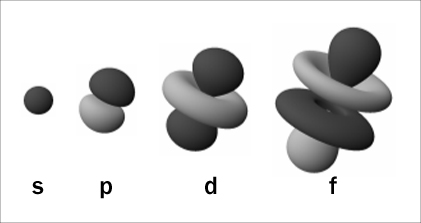
Electrons fill spaces near an atom based upon the energy required by an electron to maintain its position. The spaces requiring the least energy are filled first while those spaces requiring more energy are filled last, similar to pouring water into a glass. The lower potential energy position is the bottom of the glass, and as water continues to be poured the water rises into a position of higher potential energy. The lowest energy orbitals are called the s orbitals. The s orbitals are spherical in shape. Since only two electrons can ever occupy any orbital, the first s orbital is full when it has two electrons in it. This also corresponds to the element He, which you notice, is at the end of row 1. The next row begins filling orbitals by using the spaces that require the least energy first, s orbitals require the least energy, and so the first orbital in the second row is an s orbital. There are eight elements in the second row and each element must have all of its electrons placed in space. The second energy level (row two) beginning with lithium, contains two types of orbitals s and p, where the p orbitals resemble a dumbbell shape with orientations that are physically as far separated from one another as possible. The three p orbitals are designated by X, Y, and Z which represent the three perpendicular axis. Each p orbital can hold 2 electrons meaning the total number of electrons in the p orbit is 6. The two electrons in the s, plus the six electrons in the p orbital give a total of eight electrons, the rule of octet. The three p orbitals are shown below.
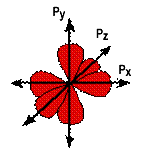
The periodic table not only has relationships within the horizontal rows (periods) but also within the vertical rows (or groups). The first two vertical columns, groups 1A and 2A, are elements whose s orbitals are filled. For any given orbital, it requires two electrons (with opposite spins) to fill the orbital space. For example,
Each atom of an element has one more electron than the previous element and a regular pattern for where that electron can be found. Within the increasingly complex three-dimensional spaces that these electrons occupy, electron configuration can be determined fairly readily by simply locating the element on the periodic table.
Elements in vertical columns (groups) 3A through 8A fill their p orbitals. Remember that you can have a p orbital on the x axis, the y axis, or on the z axis (all being able to hold 2 electrons with opposite spins). For example, an atom of the element nitrogen has two energy levels with two electrons in the first energy level (in an s orbital), two electrons in the second energy level (in an s orbital), and three electrons in p orbitals in the second energy level—one in the x direction, one in the y direction, and one in the z direction. Remember the energy requirement. Once an electron occupies a space in one of the p orbit lobes, that lobe has a higher energy requirement for new electrons than a completely un-occupied lobe.
Locate the atoms of elements whose energy levels contain d orbitals, also known as the transitional elements. These orbitals are more complex than the p orbitals with five different possible orientations. The complexity of the electron arrangement accounts for properties of the elements such as iron, copper, nickel, and zinc, which can exist in many different oxidation states. Iron is a common metal that not only changed the history of man (the Iron Age), but also was responsible for an industrial boom in American history, beginning with the growth of the railroad in the 1860s and peaking one hundred years later. One of the most reliable measures of a country’s national economy was its steel production throughout much of this period. The electron arrangement of iron makes it perfect for alloying in various ratios for its industrial uses, and also ideal as the central atom in hemoglobin, responsible for carrying oxygen in many animals. The d orbital arrangement of electrons in an element such as iron is what gives it the unique characteristics for its reactions in nature.
The most complex orbitals are the f orbitals: they have seven different orientations. These orbitals are found in the lanthanides and actinides—see if you can find them. These elements are commonly unstable, many of them are radioactive, and some do not even exist in nature, having only been synthesized in particle colliders. Two of the most commonly known elements in this group are uranium and plutonium, both naturally occurring. The potential uses of these very complex elements are a constant source of anxiety in world politics because of their unique and dangerous characteristics. One kilogram of plutonium 239 can be converted to produce about 22 million kilowatt hours of heat energy.
Keep in mind that these areas for finding electrons with the greatest probabilities are based on mathematical equations, and experimentation to even confirm that an electron did exist in a location required hitting it with another electron to knock it out of the space. Heisenberg’s uncertainty principle was based on this relationship. The German chemist Werner Heisenberg won the Nobel Prize in 1932 for his contributions to atomic structure, and his uncertainty principle is an important contribution for all scientists. To measure the existence of the electron required changing the physical structure of the atom he tried to measure. This idea of simply measuring something that changed what you are trying to measure has been extended to other areas of science as well. Being aware of your bias (the fact that you always affect what you are studying) is critical to elegant experimental design. This has obvious implications across all of the scientific fields that require experimentation to test hypotheses. Understanding that simply studying something involves an inherent change to the system can be an important breakthrough.