 username@email.com
username@email.com
In this lesson, you will review the basics of biology.
Electrons do not orbit the nucleus like planets orbit in our solar system. Instead, electrons can be anywhere in a cloud-like region around the nucleus of an atom. This “cloud” is a visual model that symbolizes where electrons are likely to be found. An electron’s movement is related to its energy level, or the specific amount of energy it has. Electrons of different energy levels are likely to be found in different places. For example, lower energy electrons are close to the nucleus and higher energy electrons are farther away from the nucleus.
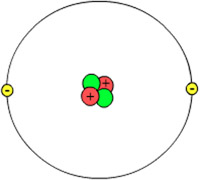
This helium atom shows a nucleus with two protons (red) and two neutrons (green). Its probable electron “cloud” (gray) shows two electrons (yellow).
An atom’s valence electrons are those that have the highest energy level and are held most loosely and farthest from the nucleus. The number of valence electrons in an atom of an element determines the chemical properties of that element, including the ways in which the atom can bond with other atoms.

A molecule of methane that shows a covalent bond of hydrogen and carbon. Red depicts an electron from carbon. Green depicts an electron from hydrogen.
Atoms with five, six, or seven valence electrons become more stable when this number increases to eight. So when they react, they tend to gain electrons from other atom(s). Similarly, atoms with one, two, or three valence electrons can lose electrons to other atom(s) so they have eight in their previous level, and become more stable. Atoms become more stable by having their outermost electron cloud either completely full (often eight) or empty of electrons. When these two types of atoms combine, electrons are transferred from one atom to another, making the atoms more stable and able to bond tightly together in an ionic bond. The other type of chemical bond occurs when it is easier for two or more atoms to share electrons and is called a covalent bond. A covalent bond is formed when the total number of shared valence electrons completely fills or empties the outermost electron level. Covalent bonds usually form between atoms of non-metals, whereas ionic bonds usually form between a metal and a non-metal.
Valence electrons are found in
The correct answer is B. An atom’s valence electrons are those that have the highest energy level and are held the most loosely and farthest from the nucleus. One of the most important features of an element’s reactivity is how many valence electrons (or space for donations) are available to form bonds with other atoms.
Covalent bonds are mostly responsible for holding hydrogen and oxygen atoms together in a water molecule. In covalent bonding, the atoms sometimes have a partial electrical charge (positive or negative), because of the unequal sharing of the electrons. Often one of the atoms will draw an unequal share of the electrons around it leaving the other atom with a smaller amount of electrons. The atom that draws the most electrons also receives their negative charge giving that part of the atom a partial negative charge. The other atom has fewer electrons around it, so it has a slight positive charge provided by the protons in the nucleus, and absence of electrons in the orbitals. This type of compound is called a polar compound because it has a slight positive and a slight negative region. Water is a polar compound because the oxygen atom tends to pull an uneven distribution of the electrons away from the hydrogen atoms leaving the hydrogen atoms with an electron deficit. Therefore the oxygen side of the water molecule is slightly negative, due to the overload of electrons while the hydrogen side has a slight positive charge due to the lack of electrons. Interestingly, the negative charge of the oxygen side of the molecule attracts the hydrogen side of neighboring water molecules (opposites attract) creating the cohesive properties of water, like surface tension.
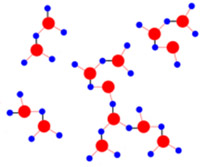
Hydrogen bonding between water molecules is represented by black lines. Covalent bonds between hydrogen (blue) and oxygen (red) are represented by red lines.
In water, the oxygen is a bit more negative and the hydrogen atoms are a bit more positive. The opposite charge causes the hydrogen atoms of one water molecule to be pulled toward the oxygen atom of nearby water molecules, creating a strong non-covalent bond to hold the water together. This strong bond, called a hydrogen bond, makes it difficult to boil or freeze water. This is important because living organisms contain so much water. The covalent bond strength means that water is a stable molecule; it is very hard to pull oxygen and hydrogen of a water molecule apart into the atoms. Although water contains covalent bonds, the characteristics of boiling and freezing water are due to its network of hydrogen bonds, not its covalent bonds.
What chemical bond is formed when two atoms share electrons?
The correct answer is C. Metallic bonds possess positive ions in a sea of electrons that move freely in all directions within a metal. An ionic bond is one in which one atom gains a valence electron from another atom. Valence refers to electrons that reside in the outermost electron shell of an atom and interact with each other.
One of the most important classes of molecules of life is the nucleic acid. Nucleic acids are large, organic molecules made of carbon, oxygen, hydrogen, nitrogen, and phosphorus. The most familiar nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA and RNA are made of small molecules connected in a now-familiar pattern of a spiral ladder or double helix.
The building blocks of the nucleic acids are called bases that form bonds with one another to stabilize the double helix structure of DNA. These base pairs are the crucial information-laden aspect of DNA that empowers the molecule to be able to handle all genetic instruction for the development of all life as we know it. There are four bases that make up DNA: Adenine (A), Cytosine (C), Guanine (G), and Thymine (T). The arrangement of these four bases are the primary variables in DNA—the molecule of heredity.
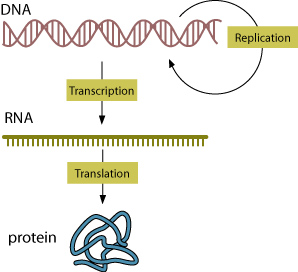
In even the simplest forms of life, DNA can contain millions of nucleotides that encode all the information the organism needs to live. RNA is also built of just four kinds of nucleotides, but RNA and DNA molecules are slightly different in form: RNA is a single strand, DNA is a double strand; in RNA, Uracil (U) replaces Thymine (T). DNA and RNA also function differently. Think of RNA as both an evolutionary precursor to DNA and as DNA’s helper in protein synthesis.
The diversity of life owes its existence (at least in part) to the number and order of nucleotides in DNA (and RNA). DNA consists of a double-stranded chemical instruction manual for everything an organism needs to do: grow, divide, even when and how to die. RNA is usually single-stranded and carries instructions usually to ribosomes for creation of specific proteins. The order of DNA nucleotides determines a related sequence in RNA. The sequence of RNA nucleotides, in turn, determines the amino acids in proteins made by the ribosomes of a living cell.
Just as all the pieces in a puzzle have individual roles in making the final picture, all organisms have an individual role in the movement of energy through their ecosystem. An organism’s energy role is determined by how it obtains its energy and interacts with other organisms. Each organism in an ecosystem fills the role of a producer, consumer, or decomposer.
The sun provides energy to most ecosystems. Some organisms, such as plants, algae, and some bacteria, capture the sun’s energy and store it as food energy for later use. Some organisms on the deep sea vents utilize other forms of non-solar energy, and make a living that way. Other organisms store food energy as fat or other biomolecules for later use. This kind of energy is called potential energy—food energy stored and held for later use by an organism. The stored energy has a chemical potential for providing energy at a later time, much like potential energy in a physical science application. Organisms use the stored energy from the sun to convert water and carbon dioxide from the environment into food (sugar) molecules via photosynthesis and consume the energy to live. The energy being actively used by a plant or animal is called kinetic energy, much like energy of motion in a physical science application.
Living organisms follow the same rules of physics that all objects do. With regards to energy, every thing and every organism is governed by the laws of thermodynamics. Thermodynamics is the study of the interaction between heat (thermo) and energy (dynamics).
The first law of thermodynamics states that energy can neither be created nor destroyed. This law applies to the chemical processes in all organisms. It says that the total energy of a system of an organism and its surroundings stays the same, although the form of the energy may change or the system may gain or lose energy to the surroundings. If an organism has stored energy, that energy may go to produce both motion and heat energy in the organism as it moves or performs some task. That expended energy in turn is released to the surroundings. Although energy has been transformed from one form to another, the total amount of energy between the organism and its surroundings always stays the same.
Although the classic egg-in-a-bottle experiment is over 100 years old, it was not made widely popular until the late 1960s by an Australian physicist, Professor Julius Sumner Miller. The video link below connects you with this classic thermodynamics experiment that can easily be demonstrated in the classroom.
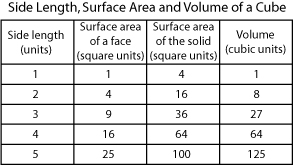
The relationships among surface area, volume, and mass are an integral aspect of the physical properties of all biological systems. A primary law of how organisms relate to their environment has a lot to do with their size, surface area, and mass. Great examples are the limits of certain body types, organs, and cells. The table below shows how the volume of an object increases relative to surface area and length. As the length of a side increases, the surface area increases as the square; the volume increases as the cube of the surface area increases in linear dimension.
There is a mutation in a population of insects that doubles the length of wings from one unit to two units. Assuming the new wings are the same size, how will this addition affect the surface area?
The correct answer is D. We know that when a linear dimension of an object doubles, its volume increases by the cube. Since the cube of two is eight, the mutated insect would weigh eight times as much as the non-mutated insect.
Think of how and why surface area to volume ratios can be integral aspects of the development of organ systems like our digestive, reproductive, respiratory, and circulatory systems. Think of why there are limits to the sizes of organisms. Could this also be related?
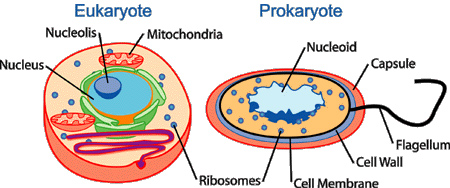
There are two types of cells—prokaryotes and eukaryotes. Prokaryotic cells preceded eukaryotic cells by about two billion years. Prokaryotic cells first appeared about 3.5 billion years ago; eukaryotic cells showed up about 1.5 billion years ago.
Eukaryotic cells do one additional process: cell differentiation. This occurs during embryogenesis when unspecialized cells differentiate into specialized cells.
Prokaryotic cells do not have a true nucleus; instead, their nuclear structure is called a nucleoid that contains genetic material in a single, circular DNA molecule. Additionally, prokaryotes are cells without a membrane-bound nucleus or organelles. The exterior of the prokaryotic cell usually has structures for locomotion, such as glycocalyx, flagellum, fimbriae, or pili. Bacteria are prokaryotic cells.
Eukaryotic cells are larger and more complex than prokaryotic cells. Eukaryotes have a membrane-bound nucleus containing chromosomal DNA. Eukaryotes also have highly organized, membrane-bound organelles, each performing a specialized function. Many eukaryotic cells have a cell wall. Animals, plants, algae, fungi, and protozoa are eukaryotic cells.
Some differences between prokaryotic and eukaryotic cells are as follows:
| Prokaryotic and Eukaryotic Cell Differences | ||
|---|---|---|
| Characteristics | Prokaryotic | Eukaryotic |
| Cell wall | Chemically complex | Chemically simple |
| Plasma membrane | No sterols or carbohydrates | Sterols and carbohydrates |
| Glycocalyx cell wals | Contain a capsule or slime layer | Contained in cells without |
| Flagella | Protein building blocks | Multiple microtubules |
| Cytoplasm | No streaming | Streaming |
| Membrane-bound organelles | No | Yes |
| Nucleus | No | Yes |
| Chromosomes | Single, circular | Single, circular |
| Cell division | Binary fission | Mitosis |
| Sexual reproduction | No meiosis | Meiosis |
Which of the following is an example of a prokaryotic cell?
D is the correct answer. Choices A and C are examples of eukaryotes. Choice B is neither prokaryotic nor eukaryotic.
Which of the following is a difference between prokaryotic and eukaryotic cells?
C is the correct answer. Choices A, B, and D are processes shared by prokaryotes and eukaryotes.