 username@email.com
username@email.com
⬅ Previous Lesson Workshop Index Next Lesson ➡
In this lesson, you will review chemical reactions; the concept of the mole as a unit of measurement and how to use it; the properties of chemical solutions and how they are classified; and how absorption or energy release are related to chemical reactions.
Matter is constantly changing. The food that you eat becomes part of your body; gasoline becomes carbon dioxide, water, heat, and the energy that runs your car; the ink in your printer cartridge binds with paper to form a document. The process by which atoms of substances rearrange to form new substances is called a chemical reaction. Some indications of a chemical reaction include a change in temperature, such as in fire, a color change that indicates the presence of a different substance, and the generation of electrical current by a battery. Chemical reactions are written using shorthand similar to a mathematical equation:
reactant A + reactant B → product C + product D
But there are many different types of reactions. For example, burning hydrogen in oxygen can be written as:
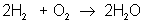
You can see that the equation for the reaction of hydrogen and oxygen indicates more than just the reactants and products. It further shows that two molecules of hydrogen react with a single molecule of oxygen to produce two molecules of water. The total number of hydrogen atoms (four) and the total number of oxygen atoms (two) are the same on both sides (reactants and products) of the reaction. Unlike nuclear reactions, chemical reactions do not change the identity of any atom. There are always the same numbers and types of atoms before and after the reaction; they are just arranged differently. Although new combinations of molecules exist, the equation must be balanced to show all atoms are accounted for.
Equations are balanced by changing the number of molecules in the equation. Coefficients, which indicate number of units, can be changed. Subscripts that identify the number of atoms within a molecule, however, cannot be changed.
In order to balance an equation, we start with a basic equation that shows all the reactants and the products. For example:
ethane + oxygen → carbon dioxide + water
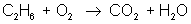
reactant: C – 2 ; H – 6 ; O – 2
product: C – 1 ; H – 2 ; O – 3
Adjust coefficients so that carbon is balanced on both sides:
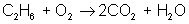
reactant: C – 2 ; H – 6 ; O – 2
product: C – 2 ; H – 2 ; O – 5
Next, adjust coefficients so that carbon is balanced on both sides:
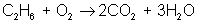
reactant: C – 2 ; H – 6 ; O – 3
product: C – 2 ; H – 6 ; O – 7
Then adjust coefficients so that oxygen is balanced on both sides:
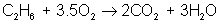
reactant: C – 2 ; H – 6 ; O – 7
product: C – 2 ; H – 6 ; O – 7
And finally, make all coefficients whole numbers:
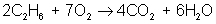
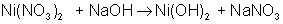
balances to:
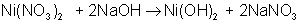
There are millions of different chemical reactions that occur everyday all around us. Chemists organize information about reactions by classifying them by patterns of chemical change. This classification helps predict the products and conditions of a chemical change. Most chemical reactions fall into four categories:
Synthesis Reaction is a reaction in which two or more reactants combine to form a single product (also called a composition reaction):
A + B → C
An example of a synthesis reaction is:
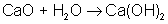
AB → A + B
An example of decomposition is the breakdown of hydrogen peroxide when it is exposed to light:
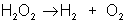
AB + C → A + BC
An example of a single-displacement reaction is:
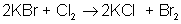
AB + CD → AC + BD
An example of a double-displacement reaction is:
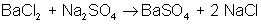
Which of the following is a synthesis reaction?
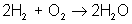
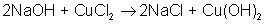
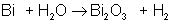
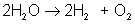
The correct choice is A. B is a double-displacement reaction; C is a single-displacement reaction; D is a decomposition reaction.
Chemical equations express chemical changes in terms of a specific ratio of atoms, molecules, or ions. Remember that all of these particles are extremely small, so we never observe the reaction between one atom of A and one atom of B. In fact, scientists rarely work in units of hundreds, or even billions of atoms. In the SI system, the base unit for measuring an amount of material is the mole (abbreviated mol). A mole is the number of carbon atoms in exactly 12 grams of pure carbon-12. The mole has been determined experimentally (although no one has actually counted a mole of anything) as 6.0221367 × 1023, also known as Avogadro’s number. It is generally rounded to three or four significant figures — 6.022 × 1023. Because it is a unit of quantity, a mole can apply to anything—one mole of atoms, one mole of ions, even one mole of moles (the animal). In reality, the number is so big that it finds little use outside of chemistry and related fields. (One mole of moles would cover the whole planet with a layer of animals tens of kilometers deep.)
Avogadro’s number is very useful in chemistry. One mole of any substance weighs as many grams as its atomic mass. Therefore, the mass of one mole of a pure substance, called its molar mass, is equal to its atomic mass with units of grams/mole. The atomic mass of oxygen, for example, is 16.0 and its molar mass is 16.0 g/mol. The molar mass of water is 18.0 g/mol, so if you measure out exactly 18 grams of water (or ice), you will have 6.02 × 1023 molecules in a beaker.
Avogadro’s number can also be used to determine the volume of a gas. Avogadro was the first to propose the principle that equal volumes of all gases at the same temperature and pressure contain the same number of particles. The molar volume for a gas is the volume that one mole of gas occupies at 0.00°C and 1.00 atm pressure, conditions known as standard temperature and pressure (STP). This volume is 22.4 liters for every gas. That means that 22.4 liters of hydrogen at STP will have a mass of 2.00 grams (remember that hydrogen has diatomic molecules), while the same volume of helium has a mass of 4.00 grams. The conversion factor, 22.4 L/mol, allows you to calculate the number of particles in a given volume of gas at STP or, combined with the molar mass of a substance, this conversion factor can be used to determine the mass of a given volume of gas.
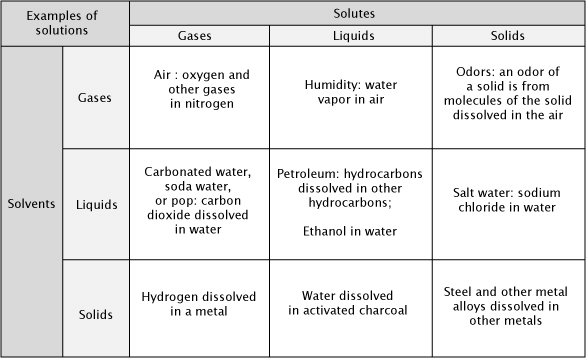
We are surrounded by solutions—and we are composed of solutions. A solution is a homogeneous mixture containing two or more substances. It is impossible to distinguish one substance from the other in a solution, which can be a gas, liquid, or solid. The air you breathe is a solution that contains oxygen, carbon dioxide, water vapor, and several other gases dissolved in nitrogen. Most of the matter inside of your body is filled with solutions of numerous substances in water. The major component of a solution — nitrogen or water in these examples — is the solvent. The substance that forms a solution in the solvent is the solute.
Water is the most common solvent of liquid solutions and is referred to as the universal solvent. Almost all substances dissolve in water. The process of forming a solution depends on interactions between the particles — atom, ions, or molecules — of the solvent and the solute. Because of the shape of water molecules and the tendency of electrons to spend more time around the oxygen atom than around the hydrogen atoms, one side of the molecule is slightly electrically positive and the other is electrically negative. Water molecules are polar. Other substances that are also polar tend to dissolve more easily in water than substances that are nonpolar. As shown below, many ionic compounds dissociate in an aqueous (water) solution. The negative ions are attracted to the positive, or hydrogen, end of the water molecule and positive ions are attracted to the negative end. Nonpolar molecules, which have no separation of charge, such as the components of olive oil, dissolve well in nonpolar solvents, but not in water.
Sometimes a solution can contain more solute than a normal saturated solvent at a particular temperature. This supersaturated solution normally occurs when a saturated solution is formed and then cooled. As the solubility decreases, the solute can stay in solution at a concentration higher than its normal solubility limit. If the supersaturated solution is disturbed, the excess solute comes out of solution as a solid, a process called precipitation. Supersaturated solutions can also be formed when some of the solvent evaporates.
The concentration of a solution is a measure of how much solute is dissolved. Concentration can be expressed quantitatively as a ratio of the amount of solute to the amount of solvent, or the total amount of solution. Chemists frequently use the ratio of mass of solute or the number of moles of solute per volume of solution. The molarity of a solution, indicated by the symbol M, refers to the moles of solute/liter of solution.
Example: 50.0 g NaCl in 500 milliliters of solution
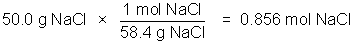
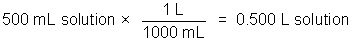
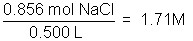
Example: 450 ml of a 2.50M solution of LiBr
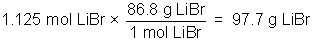
The chemical properties of a solution depend on how the solvent and solute interact with other substances. However, there are some properties of solutions that are affected by the number of particles in solution, regardless of identity of the solute. These colligative properties are a function of concentration. When a covalent compound, such as sucrose (sugar), is dissolved in water, the effect of the colligative properties is determined by the molarity of the solution. Ionic compounds, however, dissociate into ions, so the number of particles is a multiple of the molarity of the compound in solution. This means that a 1 M solution of sodium chloride has the same effect as a 2 M solution of sucrose because there are twice as many particles in solution. Colligative properties include:
All aqueous solutions contain hydrogen ions (H+) and hydroxide ions (OH–). The relative amount of these two ions determines whether a solution is neutral, acidic, or basic. In a neutral solution, the concentration of the two ions is identical. In addition to pure water, solutions of salts such as NaCl, are neutral. An acidic solution contains more hydrogen ions than hydroxide ions; a basic solution contains more hydroxide ions than hydrogen ions. This ratio depends on the composition of the dissolved substance and how it interacts with water.
There are many different compounds that form acidic or basic solutions. Common acids include foods, such as orange juice and vinegar, as well acids that are dangerous to handle, such as the solution in automobile batteries. Common bases include antacid tablets that settle your stomach and drain cleaner that can dissolve clogs in sewer pipes.
General properties of acids include:
General properties of bases include:
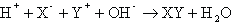
Notice that the final property listed for acids and bases is that they react with one another to form a salt and water. This process is known as neutralization. The general form of a neutralization reaction is a double-displacement between an acid (HX) and a base (YOH), which can be written as:
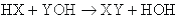
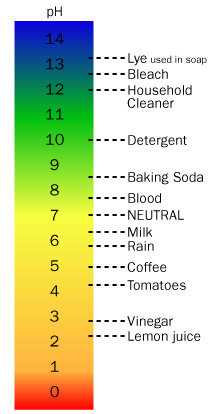
The relative strength of acids and bases is measured on a scale known as the pH scale. The pH is the negative logarithm of the hydrogen ion concentration. That means the pH of an acidic solution, with a hydrogen ion concentration of 1 × 10-1 M is equal to 1. The pH of a basic solution with a hydrogen ion concentration of 1 × 10-13 M is equal to 13. Solutions with very low pH values, such as 1 or 2 are considered strong acids, while solutions with a pH value of 5 or 6 are weak acids. Strong bases have high pH values, such as 13 or 14, while weak bases are solutions whose pH is in the 8 to 9 range. The strength of an acid or base solution depends on the concentration of the solute and to what extent it dissociates to form hydrogen or hydroxide ions in solution.
An aqueous solution has a hydrogen ion concentration of 1.0 × 10-6 M. This solution is classified as a
The correct answer is B. pH = -log[hydrogen ion concentration] = -log ( 1.0 × 10-6 ) = 6. A pH value of 6 indicates a weak acid.
Energy is the ability to do work or produce heat. Every chemical substance has kinetic energy, based on the motion of its atoms and molecules, as well as stored energy known as chemical potential energy. The potential energy is energy that was added to the substance as bonds formed and it interacted with other atoms and molecules. Chemical changes can release this stored energy as heat, the process of energy flow. Every chemical change either releases or absorbs energy. A process that absorbs energy is called endothermic; a process that releases energy is called exothermic.
The change in potential energy during a chemical reaction can be illustrated in an exothermic energy diagram that shows the change in potential energy. In an exothermic reaction, the energy of the reactants is higher than that of the products. The change, released as heat, is indicated as Delta E in the diagram. Notice that initially some energy must be added to start the reaction. This energy, labeled E sub a is called the activation energy of the reaction. Once the reaction starts, activation energy is provided by the reaction itself. An example of activation energy in an exothermic reaction is the striking of a match. The match does not start to burn until a small amount of energy is added by the friction of the match against rough side of the box.

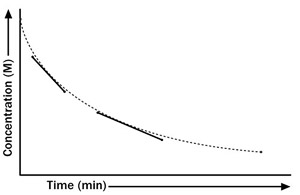
The rate of a reaction is the amount of change from reactant to product over a given period of time. When these quantities are plotted on a graph, the slope of the line indicates the reaction rate. The rate of a chemical reaction is increased by adding a catalyst that reduces the activation energy, thereby lowering a barrier to the chemical reaction. For example, hydrogen peroxide breaks down to form hydrogen and oxygen. The rate of reaction can be measured by measuring the amount of oxygen produced. Adding a catalyst, such as many transition metals, causes hydrogen peroxide to break apart faster.