 username@email.com
username@email.com
In this lesson, you will review lipids.
Lipids all contain long chains or rings of hydrocarbons that give them their relatively hydrophobic and non-polar characteristics. Examples of lipids include fats and oils, waxes, phospholipids, and ringed steroids, such as cholesterol and steroid hormones. Lipids are important components of cell membranes. They serve as a form of long-term energy storage, act in transport, and function as chemical messengers.
Fats and oils are triglycerides, esters of glycerol, and fatty acids. They are formed in dehydration synthesis reactions. Triglycerides are the primary form of long-term energy storage in plants and animals. Fatty acids are long chains of 12 to 24 hydrocarbons with a carboxyl group at the end. Bonding within fatty acid chains gives fats and oils their characteristics.
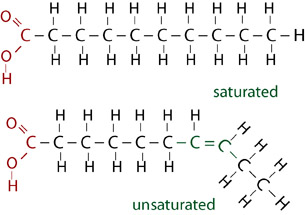
Fats and oils can be classified as saturated, monounsaturated, or polyunsaturated, depending on the number of double bonds between carbon atoms in the fatty acid chains.
Animal fats have all single-carbon bonds and so are bonded to the maximum number of hydrogen atoms possible. The single bonds form a straight fatty acid chain. They are called saturated fats and are solid at room temperature.
Oils have some double bonding of carbon atoms and so are unsaturated fats. The double bonds create bends in the fatty acid tails, which makes unsaturated fats liquid at room temperature.
Polyunsaturated fats have multiple double bonded carbons.
Waxes are also esters of long chain fatty acids bonded to long chain alcohols. Waxes function to provide a waterproof coating on many living things. Plant cuticles are waxy, as are many of the oils secreted by the skin. The exoskeleton of insects contains wax.
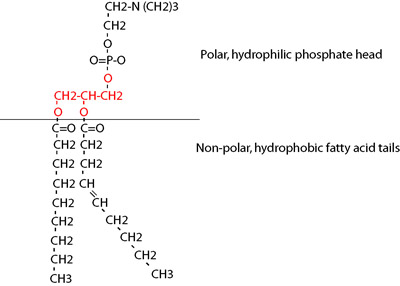
Phospholipids are composed of glycerol, two fatty acids, and a phosphate head. The fatty acid tails of the molecule are hydrophobic, while the phosphate end of the molecule is polar and hydrophilic. This arrangement is ideal for function in double-layered cell membranes. In solution and in cells, the polar heads of phospholipids arrange themselves opposite each other, creating an oily membrane interior. The unique structure of phospholipid bilayers makes them selectively permeable.
Steroids all contain a core consisting of four fused hydrocarbon rings. Examples of steroids include cholesterol, steroid hormones, and vitamins. Steroids are differentiated by the unique groups bonded at the outer regions of the four ring core.

Hormones are chemical signals that act only on cells with specific receptors. Steroid hormones include the sex hormones estrogen, progesterone, and testosterone. Cortisone is also a steroid hormone, which is important in carbohydrate metabolism. It has anti-inflammatory properties that make it important in treatment of some diseases. A group of similar steroids acts as vitamin D.
Lipoproteins are composed of proteins and several types of lipids bonded together. Some lipoproteins serve as catalysts, and many are associated with membrane-bound proteins. Some lipoproteins function to transport other lipids, such as cholesterol and steroids, throughout the body. Lipoproteins are classified based on their density. High-density lipoprotein (HDL) transports cholesterol to the liver. Low-density lipoprotein (LDL) transports cholesterol to body tissues. Very low-density lipoprotein (VLDL) transports triglycerides for storage as fat.