 username@email.com
username@email.com
In this lesson, you will review nucleotides and nucleic acids.
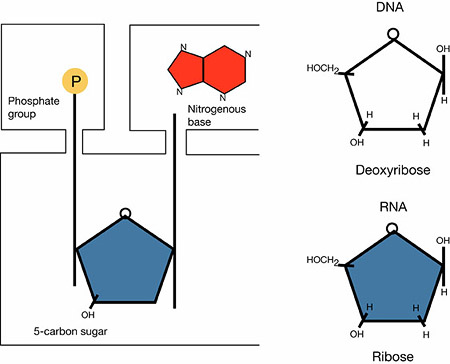
The nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), carry the instructions for the structure and function of all living things. Those instructions are stored in the cell nucleus and passed from generation to generation via DNA. The DNA instructions are carried by the RNA to the appropriate site, usually a ribosome, and used to build proteins as determined by the original DNA. It is thought that life began when a strand of RNA became incorporated in a cell membrane with proteins.
Nucleotide monomers contain a five-carbon sugar called a pentose, one or more phosphate groups, and a nitrogenous base. The pentose sugar of RNA is ribose; its counterpart in DNA is deoxyribose. When nucleotide monomers form phosphodiester bonds the phosphate of one monomer bonds to the sugar of another, forming the backbone of the nucleic acid molecules.
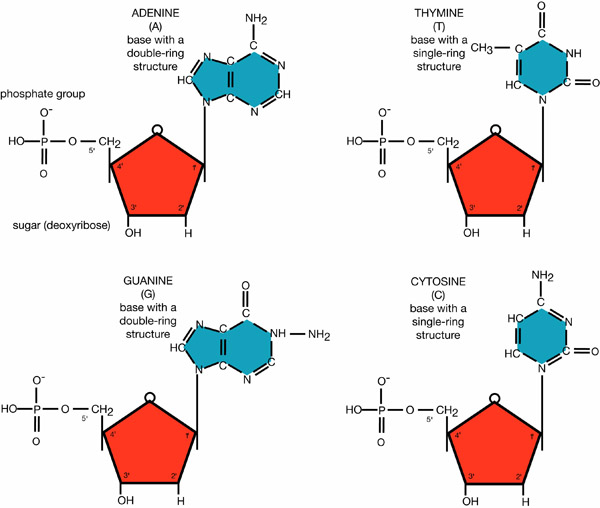
Each monomer contains one of five nitrogenous bases. They are classified as either pyrimidines or purines. The three pyrimidines—cytosine, thymine, and uracil—are single-ring structures. The purines—guanine and adenine—are double-ring structures. Adenine, guanine, and cytosine all occur in both DNA and RNA molecules. Thymine is unique to DNA and uracil is unique to RNA.
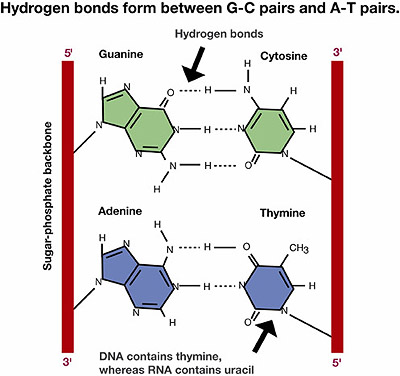
RNA is a single-stranded molecule, while DNA is double-stranded. The double-stranded structure of DNA comes from hydrogen bonding between pyrimidines and purines. The hydrogen bonded nitrogen bases form the rungs of the DNA double helix ladder. In DNA, adenine always forms two hydrogen bonds with thymine, and cytosine always forms three hydrogen bonds with guanine. In RNA, uracil pairs with adenine. No other hydrogen bonding configuration between bases is possible.

The double helix of DNA is composed of a coding strand and a template strand. The cell uses the template strand in the nucleus to build complementary strands of RNA. Single-stranded RNA carries the code in complementary bases to the site of protein production in the cell cytoplasm.

Other organic molecules are important in cell metabolism and in enzyme regulation. Several nucleotides (NAD and NADP) are important coenzymes in photosynthesis and respiration. Many are important in substrate level phosphorylation reactions.
Adenosine triphosphate (ATP) is the form of energy cells use to do work. ATP is the form of energy plants produce during the light reactions of photosynthesis. It is the form of energy released during cellular respiration. Each ATP molecule is composed of a ribose, adenine, and a chain of three phosphate molecules. Because the phosphates have a negative electrical charge, they tend to repel each other. It takes energy to maintain those bonds; when they are broken, energy is released.
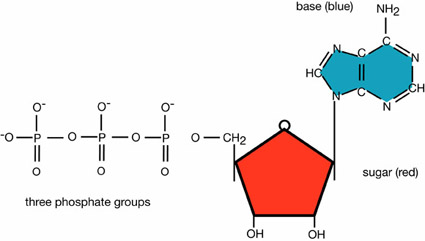
When the endmost oxygen-phosphate bond is broken, energy is released, and adenosine diphosphate (ADP) and a free phosphate are produced. Cells use energy to form the bonds in ATP and release it when those bonds are broken. By continuously cycling ADP and ATP, cells efficiently transform energy.
How does DNA differ from RNA?
B is the correct answer, because DNA is double-stranded, contains deoxyribose, and has plenty of phosphodiester bonds. RNA is single-stranded and contains ribose.