 username@email.com
username@email.com
In this lesson we will review cell potential and how to calculate emf. We will also review the relationships between cell potential, work, and free energy.
Cell potential is the driving force in electrochemical cells that moves the electrons from the substance being oxidized to the substance being reduced. Cell potential is measured in volts (V) and is the difference between the electric potential of two electrodes.
Cell potential also indicates whether a reaction is spontaneous under standard conditions (1 atm pressure, 298 K, 1.0 M solutions). A negative value of cell potential means the reaction is not spontaneous, while a positive value is spontaneous under standard conditions. Should the reaction in an equation need to be reversed, the sign of the value of the reduction potential will need to be reversed as well.
Tables of standard reduction potential (E°) contain given values for reactions that undergo the reduction process in redox half-reactions, thus gaining electrons, or becoming more negative. Values of standard reduction potential are measured at 25°C, 101.325 kPa, and the solutions’ concentrations are 1 M. Tables of standard reduction potential are determined using hydrogen half cells. For a hydrogen half-cell to work it must be connected to another half-cell. This will make a voltaic cell. By definition, the potential of the hydrogen half-cell is 0.00 Volts, so the voltage measured in the voltaic cell must be due to the other half-cell. Therefore, when the hydrogen half-cell is connected to another half-cell it is possible to determine the potential of the other half cell.
| Standard Reduction Potential | |
|---|---|
| Reaction | E° (V) |
| Be2+(aq) + 2e– ↔ Be(s) | -1.97 |
| Al3+(aq) + 3e– ↔ Al(s) | -1.68 |
| Mn2+(aq) + 2e– ↔ Mn(s) | -1.18 |
| Fe2+(aq) + 2e– ↔ Fe(s) | -0.447 |
| Pb2+(aq) + 2e– ↔ Pb(s) | -0.125 |
| Cu2+(aq) +2e– ↔ Cu(s) | + 0.340 |
| Br2 (l) + 2e– ↔ 2Br– (aq) | + 1.07 |
| Ce4+(aq) + e– ↔ Ce3+(aq) | + 1.72 |
The electromotive force (EMF) is the maximum potential difference between two electrodes of a galvanic or voltaic cell. This quantity is related to the standard reduction potential of a substance. Under standard conditions the emf is equal to the potential difference of the cell. If the value is negative, the reaction is not spontaneous and will not occur as written under standard conditions. The reverse reaction will be spontaneous.
Determine the emf for the following reaction and determine if it is spontaneous under standard state conditions.
Be2+(aq) + Pb(s) → Be(s) + Pb2+(aq)
Divide the reaction into the half-reactions and find the potentials from a standard reduction potential table.
Be2+(aq) + 2e– → Be(s); E° = -1.97 V
Pb2+(aq) + 2e– → Pb(s); E° = -0.125 V
Note: This value given is the reverse of the one needed, so before using this value, we reverse the reaction.
We know to do this because the Pb(s) is a reactant, not Pb2+.
Pb(s) → Pb2+(aq) + 2e–;E° = +0.125 V
Now we add the two reactions together.
Be2+(aq) + 2 e– → Be(s); E° = -1.97 V
Pb(s) → Pb2+(aq) + 2 e–;E° = +0.125 V
Be2+(aq) + Pb(s) → Be(s) + Pb2+(aq);E° = -1.84 V
Since the value is negative, the reaction is NOT spontaneous.
Another way to calculate the emf was determined by the German chemist H.W. Nernst who found a relationship between the conditions of the cell and its voltage. He determined that the emf can be calculated under non-standard conditions if the concentrations of the solutions are known.
His equation, known as the Nernst equation, is:
Determine the voltage of a cell if the concentration of the manganese(II) ion is 1.00 M and the concentration of the aluminum ion is 1.50 M.
2Al(s) + 3Mn2+(aq) → 2Al3+(aq) + 3Mn(s)
Split into the two half reactions
2Al(s) → 2Al3+(aq) + 6e– E° = +1.68 V
(value reversed from table to reflect this equation)
3Mn2+(aq) + 6e– → 3Mn(s); E° = -1.18 V
Now add the two reactions to obtain E°.
E° = 0.50 V
Substitute the values into the Nernst equation. Remember to raise the concentrations to the power of their respective coefficients in the balanced equation.
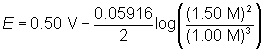
E = 0.49 V
Determine the emf of the following reaction under standard conditions.
Be + Fe2+(aq) → Fe + Be2+(aq)
Fe2+(aq) + 2e– → Fe(s);
E° = -0.447 V
Be2+(aq) + 2e– → Be (s);
E° = -1.97 V
The correct answer is C. The Be half-reaction proceeds as an oxidation as shown in the complete equation. When written as an oxidation the cell potential is reversed in sign and becomes +1.97 V. Adding +1.97 V and -0.447 V gives a cell potential of + 1.52 V.
Determine the voltage of the following cell:
Pb(s) | Pb2+(aq) (1.00M) || Cu2+(aq) (1.50M) | Cu(s)
Pb2+(aq) + 2e– → Pb(s); Eo = – 0.125 V
Cu2+(aq) +2e– → Cu(s);
Eo = + 0.340 V
The correct answer is D.
The standard potential of the cell is +0.465 V. Using the Nernst equation we substitute the concentrations of the products and the reactants.
![]()
E = 0.470 V
The work done in the cell is equal to the amount of charge in coulombs multiplied by the cell’s potential difference:
w = -qE
w represents work, q the charge in coulombs and E the cell’s potential difference.
If the cell is galvanic, the charge, q, equals the number of moles, n, multiplied by the Faraday constant, F, named for Michael Faraday. One faraday, F, is equal to 96,485 Coulombs per mole of electrons. This makes the equation become
w = –nFE.
The maximum amount of work that can be done is equal to the change in free energy, so w = ΔG. This makes the equation become
ΔG = -nFE.
Under standard conditions, the equation becomes
ΔG° = –nFE°.
The units for E° is Volts, but a volt is equal to a J/C. So the units for ΔG° will be either J or kJ.
Determine the free energy change for the following reaction:
Cu2+(aq) + Fe(s) → Cu(s) + Fe2+(aq)
Cu2+(aq) + 2e– → Cu(s); E° = 0.34 V
Fe(s) + 2e– → Fe2+(aq); E° = -0.447 V
ΔGo = –nFE°
ΔG° = – (2 mol e–)(96,485 C/mol e–)(0.787 V)
ΔG° = – 152 kJ