 username@email.com
username@email.com
In this lesson we will review how to balance chemical reactions and use mole ratios and molar masses to determine theoretical yields.
The coefficients in a balanced equation represent the number of molecules or atoms that are reacting and are produced. For example, in the formation of water, 2 molecules of hydrogen gas react with 1 molecule of oxygen producing 2 molecules of water. If 4 molecules of hydrogen gas are present, then 2 molecules of oxygen gas will be needed to produce 4 molecules of water.
2H2(g) + O2(g) → 2H2O(l)
But what if there are 400 molecules of hydrogen gas? How much oxygen gas would be required to use up all the hydrogen gas?
It can be seen that the ratio of hydrogen molecules to oxygen molecules required is always 2:1. But in the laboratory, when a measurable amount of reactants are necessary, it is advantageous to use moles to count the molecules. So 2 moles of H2 (representing 1.20 × 1024 molecules) would react with 1 mole of O2 (representing 6.02 ×1023 molecules) and produce 2 moles of H2O (representing 1.20 × 1024 molecules).
In other words, all coefficients in a balanced equation represent the number of moles of substances as well as the number of molecules, and can give a ratio between the compounds and elements in a reaction. In the case of the reaction of sodium with chlorine to produce sodium chloride:
2Na(s) + Cl2 (g) → 2NaCl(s)
The ratio of moles of Na to moles of Cl2 is 2:1, but the ratio of moles of Na to moles of NaCl is 2:2, and the ratio of moles of Cl2 to moles of NaCl is 1:2. These mole ratios can be developed for any balanced equation and can be used to determine how much product is made from a given amount of starting material.
How many moles of Cl2 would be required if 5.0 mol of Na was completely reacted?
The mole ratio of Na:Cl2 is 2:1, so we multiply the 5.0 reacted moles of Na by the mole ratio:
A chemist measures out molecules or moles not by counting, but by weighing. The molar mass of a
compound provides the connection between the mass of a sample of a substance and the number of moles of that substance in the sample.
The molar mass can be used to set up more realistic calculations from equations. For example, what if 46.0 g of sodium metal were put into a container of chlorine gas and allowed to completely react? How many grams of sodium chloride would be produced? Using a periodic table, the molar mass of sodium is found to be 23.0 g/mol. The molar mass of sodium chloride is 58.5 g/mol. This additional information is used to complete the calculation as before.
2Na(s) + Cl2(g) → 2NaCl(s)
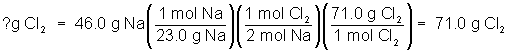
How many grams of chlorine gas are needed to completely react with 46.0 g Na?
Calculate the molar mass of Cl2 as 71.0 g/mol. Then set up the problem as before:
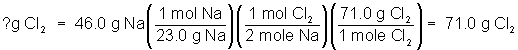
When calculating the expected amount of product from a given amount of one of the starting materials, the calculated value is called the theoretical yield. That is the amount that would be expected to be produced as calculated from the balanced equation. However, in reality, many conditions may be present to prevent obtaining the theoretical yield. For example the starting materials or product may have been impure, the recovery of the product may have been incomplete, or the reaction may have reached equilibrium before it was complete. When a reaction is finished, and the actual yield of product is determined, that amount is called the experimental yield. In order to see how effective the experimental conditions were in obtaining the desired amount of product, chemists calculate the percentage yield, or percent yield. The percent yield is the percent of the expected amount that is obtained. Thus the percentage yield is calculated by taking the actual yield and dividing by the theoretical yield, then multiplying by 100 to convert to percentage.
Often, the percent yield is less than 100%, but sometimes a perplexing percent yield of over 100% is obtained. Closer examination usually reveals a product that is contaminated with impurities or that is not completely dry.
In the production of lime, CaO, from the heating of limestone, CaCO3, carbon dioxide gas is also produced: CaCO3(s) → CaO(s)+ CO2(g).
In an experiment using 2.00 kg of limestone, 0.982 kg of lime was produced. What is the percentage yield?
The first step to getting the solution consists of calculating the theoretical yield using the information given, 2.00 kg of CaCO3 and the correct mole ratio and molar masses.

But only 0.982 kg was produced, so the percentage yield is calculated by taking the actual yield and dividing by the theoretical yield, then multiplying by 100 to convert to percentage.
![]()
So far the discussion of calculations of yields has been based on a given quantity of one starting material, assuming that there is enough of the other reactant(s) to consume the starting material in question. However, that is not always the case. Many times the amounts of both reactants are given, and they are not stoichiometrically equivalent—that is, present in the correct mole ratio for a complete reaction. In such a case, one is called the limiting reactant and the other is called the excess reactant. The limiting reactant is the one which is used up completely and thus limits the maximum amount of product that is formed. The reactant left over is called the excess reactant.
To determine the maximum amount of product, a chemical calculation must be performed as before. One of the easiest ways to determine the limiting reactant is to perform two calculations, beginning each with the amount of given reactant, and comparing the amounts of the desired product. The reactant which produced the least amount of product is the limiting reactant and this amount is the maximum of the product that can be produced.
Magnesium ribbon will react with aqueous hydrogen chloride to produce hydrogen gas and magnesium chloride.
If 15.0 g Mg(s) reacts with 0.25 mol of HCl(aq), how many moles of hydrogen gas are produced?
This is a single replacement reaction which can be written as shown:
Mg(s) + 2HCl(aq) → H2(g) + MgCl2(aq)
Comparing the amounts produced in each case indicates that no more than 0.125 mol of hydrogen gas can be produced because there is not enough HCl present to produce any more.
The hydrogen chloride is the limiting reactant and since the Mg will be in excess not all of it will react. The amount left over can also be calculated.

Moles Mg in excess would be found by calculating the moles present (0.617) and subtracting the amount reacted (0.125mol); 0.492 mole Mg will be left which corresponds to 12.0g (rounded to three significant digits from 11.9556)
What is the limiting reactant when 3.0 g of Al reacts with 5.6 g of Cl2(g) producing AlCl3? And how much of the excess reactant will be left?
The correct answer is A. That’s because the moles of Al needed to react with 5.6 g of chlorine is 0.053 mol:
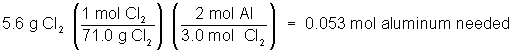
The amount of aluminum available to react is 0.11 mol:
![]()
Since aluminum is present in a greater amount than needed, the chlorine must be the limiting reactant.
To determine the amount of excess aluminum present, the amount of aluminum reacted must be calculated and subtracted from the starting amount. The number of moles of aluminum present is 0.11 moles. The amount required is 0.053 moles. The difference is 0.057 moles which is 1.5 g.