 username@email.com
username@email.com
In this lesson, we will review different types of chemical reactions and discuss how to predict the products of a reaction based upon the type of chemical reaction that will occur.
Chemical reactions occur when substances combine or decompose forming new types of chemical substances with different properties—that is, new materials are produced from the atoms that were present in the starting materials which are known as reactants. The new materials which are formed as the result of a chemical reaction are called products. Chemical equations are a short-hand way of expressing that change in matter using chemical symbols. A chemical reaction is considered balanced when there are equal numbers of each kind of atom on both sides of the equation. Because chemists believe in the law of conservation of mass, equations must be balanced in order to correctly show the changes which have occurred.
For example, when sodium metal is placed in a container with chlorine gas a chemical change occurs, as can be noted by the disappearance of the metal and the yellow-green gas and the appearance of a white crystalline solid called sodium chloride in the container. Sparks fly, heat is given off. The properties of the new material are quite different from the properties of the starting materials.
Some of the clues which signify that a chemical change has occurred are:
The chemical equation for the above reaction would be:
Na(s) + Cl2(g) → NaCl(s)
In order to balance the equation, a coefficient can be placed in front of both the Na(s) and the NaCl(s) which corrects the unbalance between the atoms.
2Na(s) + Cl2(g) → 2NaCl(s)
There are generally five different types of chemical changes that occur in matter. There are other ways to subgroup these changes in matter, but most chemical reactions can be placed into one of the following five groups:
Synthesis reactions have two or more reactants combining to produce a single product which is a compound. A new compound is created by the direct combination of reactants. The reaction of hydrogen gas with oxygen gas to produce water is such a reaction.
Decomposition reactions are the chemical opposite of synthesis reactions. In decomposition reactions one reactant, which must be a compound, comes apart to form simpler substances which may be elements or compounds. Most decomposition reactions require energy to proceed. Such reactions are also classified as endothermic. An example of a decomposition reaction is the heating of solid calcium carbonate, CaCO3(s) to form solid calcium oxide, CaO(s) and carbon dioxide gas, CO2(g).
Displacement reactions are sometimes called replacement reactions. Single displacement reactions always have an element and a compound as reactants. The element which was a reactant ends up in a compound which is a product. The element that was produced in the reaction was originally in the compound. Many of these reactions must occur in solution, but not always. If copper metal is placed in a solution of silver nitrate in a beaker, a single replacement reaction occurs. Silver metal forms in the beaker while the solution which was originally colorless begins to take on the blue color characteristic of copper(II) ion. The copper metal has taken the place of the silver ion in the solution; the silver ion becomes silver metal in the beaker. The balanced equation for this reaction is
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
Double replacement reactions, which are sometimes called metathesis reactions, are characterized by having two compounds as reactants and two compounds as products. The reactants are both ionic compounds in solution. In this type of reaction, the cations of the reactants exchange anions; the products are new compounds one of which must be insoluble, a gas or a weak electrolyte such as water. If all products are soluble ionic compounds, there will be no reaction. For example, if aqueous solutions of silver nitrate and sodium chloride are mixed, a double replacement reaction occurs producing soluble sodium nitrate and insoluble silver chloride. In equation form,
AgNO3(aq) + NaCl(aq) → NaNO3(aq) + AgCl(s)
The last type of reaction is the combustion reaction which involves the reaction of oxygen with either an element or a compound, which is usually a hydrocarbon. If the reaction is between an element and oxygen, and only the oxide of the element is formed, the reaction may also be considered to be a synthesis reaction. Typical combustion reactions of organic compounds can be recognized by the products of carbon dioxide and water. Reactants such as alcohols, carbohydrates, ketones, alkanes, alkenes and aromatics will produce water and carbon dioxide as products if the combustion is complete. If insufficient oxygen is present for complete combustion, carbon monoxide will be formed. In any case, oxygen is always a reactant.
Ethanol, C2H5OH(l), burns in oxygen to produce water and carbon dioxide according to the balanced equation:
C2H5OH(l) + 3O2(g)![]() 2CO2(g) + 3H2O(l)
2CO2(g) + 3H2O(l)
In the following list of equations, how many are replacement reactions?
2H2 + O2 → 2 H2O
Zn + CuCl2 → Cu + ZnCl2
HCl + NaOH → NaCl + H2O
CuCO3 → CuO + CO2
CH4 + 2O2 → CO2 + 2H2O
The correct answer is C. Both the second and third equations are types of replacement reactions. The second equation is a single replacement while the third equation is a double replacement reaction.
The products of reactions can be predicted by recognizing the pattern of reactants in the equation. Synthesis reactions have two or more reactants and a single product. Synthesis reactions are simply the direct combination of the reactants.
2Na + Br2 → 2NaBr
Decomposition reactions have a single reactant and several products. Decomposition reactions break down the initial compound into simpler substances which may not be the elements of the compound. For example, heating CuCO3 produces carbon dioxide and CuO not Cu, C and O2. Take time to learn some well known decomposition reactions and don’t assume that the elements are directly produced by heating a compound. Some typical decomposition reactions:
The products of single replacement reactions can be predicted because the free element, which was a reactant, is found in the compound after the reaction occurs; the element which was in the compound is replaced and is found as the free element.
If iron metal is placed in an aqueous solution of copper(II) chloride, what will the products be?
The iron is the free element and will end up in the new compound replacing the copper which will be found as a free element in the beaker. The equation can be written as Fe(s) + CuCl2(aq) → Cu(s) + FeCl2(aq) Single replacement reactions only occur if the reactant metal is more active, based on activity charts, than the one in the reactant compound. An activity chart is shown below. We will discuss more about activities of metals later in the course.
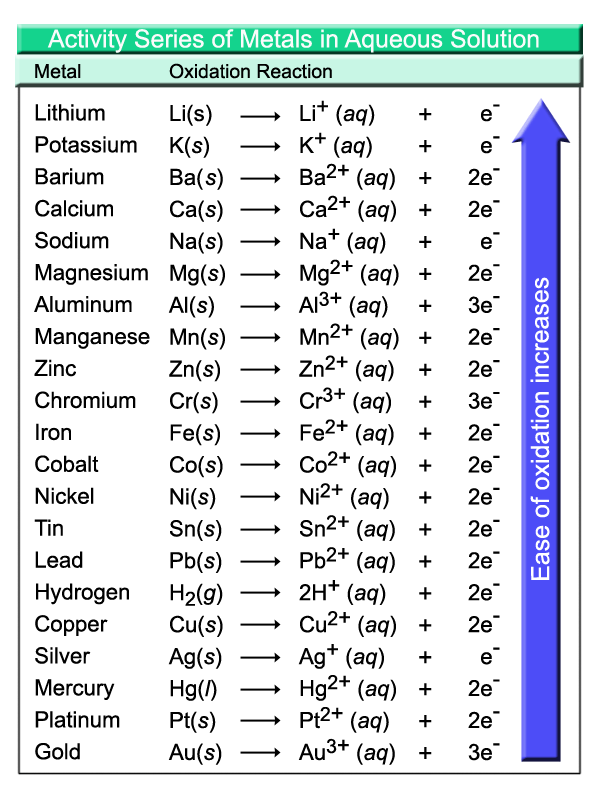
Which of the following reactions can be classified in more than one category?
The correct answer is A. That is because it is both a synthesis and a combustion reaction.
The reaction CaO + H2O → Ca(OH)2 would be classified as
The correct answer is A. That is because only one substance is produced in the reaction. In synthesis reactions, the product is always one substance.loride will not attract one another.