 username@email.com
username@email.com
In this lesson we will review how the type of bonding or attraction affects some fundamental physical properties of a substance.
Three of the most important physical properties of a compound are vapor pressure, melting point and boiling point. All three of these properties depend almost entirely upon how strongly the particles of the substance—atoms, molecules or ions—are attracted to each other. The strength of this attraction, in turn, depends upon the type of bonding exhibited by the substance in question.
Prior to looking at the properties themselves, it is helpful to look at the types of attraction that particles may have. Ionic compounds are simple, in that the ions are all interconnected in an indefinite network by ionic bonds, which are rather strong. The situation for molecular compounds is a bit more complex.
The atoms within a molecule are held together by covalent bonds, which (like ionic bonds) are strong. These are not, however, the attractions that determine the properties of interest. Instead, we are interested in intermolecular attractions. We will simplify our discussion by grouping these into two types: van der Waals attractions and hydrogen bonding.
Van der Waals attractions are caused by the presence of either a temporary or permanent dipole moment within a molecule. The slightly positive end of one molecule is electrostatically attracted to the slightly negative end of another molecule. These attractions can vary considerably in strength. Generally speaking, they are weakest when the dipole moment is temporary. They are stronger in molecules with a permanent dipole moment, and they tend to increase in strength as molecules become larger. Whichever is the case, they are still considerably weaker than either covalent or ionic bonds.
Hydrogen bonding is really nothing more than a particularly strong example of van der Waals attraction caused by a specific set of bonding conditions. It occurs when a hydrogen atom is covalently bonded to either a nitrogen, oxygen or fluorine atom. In this situation, the highly electronegative nitrogen, oxygen or fluorine pulls the shared pair of electrons away from the weaker hydrogen atom. Since hydrogen has no inner electrons (its valence electrons are on the first and only energy level, n = 1), pulling away the shared electrons exposes the hydrogen nucleus and creates a larger-than-normal dipole moment. This leads to a stronger electrostatic attraction than is typically seen in van der Waals attractions.
With the different types of attractions in mind, let’s return to the physical properties in which we’re interested. Vapor pressure at a given temperature will only increase if more particles leave the liquid or solid phase and enter the vapor phase. This can only happen when attractions are weak, so that it requires little energy for evaporation. Thus, vapor pressure is highest when attractions are weakest. Ionic compounds have extremely low vapor pressures due to the strong ionic bonds holding the condensed phase together. Acetic acid (CH3COOH) has a lower vapor pressure than does acetone (CH3COCH3). Acetic acid exhibits hydrogen bonding while acetone has only van der Waals attractions.
Melting point can be thought of as the temperature at which the particles of a substance have sufficient kinetic energy to move out of the rigid, fixed positions of the solid phase. This is also related to the strength of the attractions holding the solid particles in place. Ionic compounds are held together by strong ionic bonds, so they typically have high melting points. Molecular compounds, as a group, have much lower melting points because the molecules are held in place by the much weaker van der Waals attractions or hydrogen bonds. Stronger intermolecular attractions will result in a higher melting point.
A simplified concept of boiling point is the temperature at which a substance’s particles have enough kinetic energy to completely separate from each other and move into the gaseous state. This is still closely tied to the strength of attractions. For this reason, many small molecules with only temporary dipole moments (such as methane, CH4) boil at quite low temperatures and exist as gases at room temperature. Somewhat larger molecules with a permanent dipole moment (such as diethyl ether, CH3OCH3) exist as liquids at room temperature, but boil at only slightly elevated temperatures. Molecules with hydrogen bonding, such as water, boil at even higher temperatures. Ionic compounds, on the other hand, are held together by strong ionic bonds and tend to have extremely high boiling points.
Which of the following compounds has the highest vapor pressure at 25ºC?
The correct answer is A. Choices B, C and D all have hydrogen covalently bonded to either oxygen or nitrogen. This means that they have hydrogen bonding between molecules and are held together relatively strongly. Hexane has only van der Waals attractions, which are weaker than hydrogen bonding, so more molecules will escape to the vapor phase and raise the vapor pressure.
Which of the following compounds has the highest boiling point?
The correct answer is B. Choices A, C and D are all molecular, so they each have only weak intermolecular attractions between them. Sodium chloride is ionic, so strong ionic bonds must be overcome in order for the compound to boil. This can only occur at a very high temperature, so its boiling point is quite high.
Substances are able to conduct electricity if they have charged particles that can move throughout the substance. Ionic compounds are made of positively charged ions and negatively charged ions, but the ions can only move when the compound is molten; therefore, ionic compounds only conduct electricity in the liquid state. Molecular compounds do not conduct electricity in any physical state because molecules are electrically neutral.
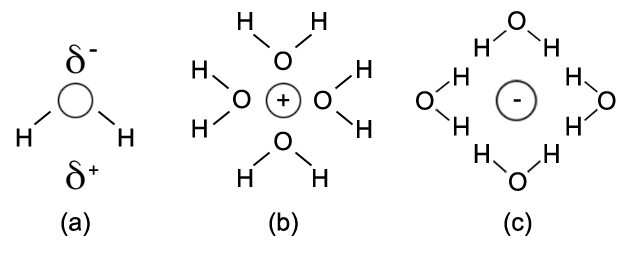
Water solubility can be a complex subject, but let’s take a simplified view. A substance can only dissolve in water if its particles are electrostatically attracted to water molecules. Since water has a large dipole moment, this occurs best with other molecules with large dipole moments, primarily those that have hydrogen bonding. This is the basis for the common expression, “Like dissolves like.” Water can also dissolve a number of ionic compounds as well, due to the electrostatic attraction between the ion charges and water’s dipole moment. The figure below shows the slightly positive (δ+) and slightly negative (δ−) ends of water’s dipole moment in part (a). Parts (b) and (c), respectively, show the interaction of water molecules with positive and negative ions when dissolving an ionic compound.
Which of the following compounds will best conduct electricity in the liquid phase?
The correct answer is C. Choices A, B and D are all molecular compounds, so they do not have charged particles. NaCl has positive and negative ions that can move freely when in the liquid state. This allows it to conduct electricity.
Which of the following compounds is most water-soluble?
The correct answer is B. The most water-soluble molecular compounds have hydrogen bonds as water does. Only choice B has hydrogen covalently bonded to either nitrogen, oxygen or fluorine, so only choice B exhibits hydrogen bonding.
Which of the following compounds is most water-soluble?
The correct answer is D. A high melting point is associated with strong attractions between particles. Choices A, B and C are all molecular compounds with relatively weak van der Waals attractions. Choice D is an ionic compound with strong ionic bonds, therefore, a high temperature is required to melt it.