 username@email.com
username@email.com
In this lesson, we will review the development of the atomic theory.

One of the first atomic theorists was Democritus, a Greek philosopher who lived in
the fifth century BC. Democritus knew that if a stone was divided in half, the two halves would have essentially the same properties as the whole. Therefore, he reasoned that if the stone were to be continually cut into smaller and smaller pieces then; at some point, there would be a piece which would be so small as to be indivisible. He called these small pieces of matter “atomos,” the Greek word for indivisible. Democritus, theorized that atoms were specific to the material which they composed. In addition, Democritus believed that the atoms differed in size and shape, were in constant motion in a void, collided with each other; and during these collisions, could rebound or stick together. Therefore, changes in matter were a result of dissociations or combinations of the atoms as they moved throughout the void. Although Democritus’ theory was remarkable, it was rejected by Aristotle, one of the most influential philosophers of Ancient Greece; and the atomic theory was ignored for nearly 2,000 years.

Although the idea of the atom was first suggested by Democritus in the fourth century BC, his suppositions were not useful in explaining chemical phenomena, because there was no experimental evidence to support them. It was not until the late 1700’s that early chemists began to explain chemical behavior in terms of the atom. Joseph Priestly, Antoine Lavoisier, and others set the stage for the foundation of chemistry. They demonstrated that substances could combine to form new materials. It was the English chemist, John Dalton, who put the pieces of the puzzle together and developed an atomic theory in 1803.
Dalton’s atomic theory contains five basic assumptions:
John Dalton’s atomic theory was generally accepted because it explained the laws of conservation of mass, definite proportions, multiple proportions, and other observations. Although exceptions to Dalton’s theory are now known, his theory has endured reasonably well, with modifications, throughout the years.
Approximately fifty years after John Dalton’s proposal of the atom, evidence began to accumulate which suggested that the atom might not be the solid sphere that Dalton had envisioned. This evidence came in the form of the discovery of electrically charged particles and radioactive materials. Based on these new discoveries, Dalton’s proposal of a solid, indestructible atom became unacceptable. Listed below, are a few of the significant discoveries that were clues that led to the development of the modern theory of the atom.
In the 1830’s, Michael Faraday, a British physicist, made one of the most significant discoveries that led to the idea that atoms had an electrical component. Faraday placed two opposite electrodes in a solution of water containing a dissolved compound. He observed that one of the elements of the dissolved compound accumulated on one electrode, and the other element was deposited on the opposite electrode. It was clear to Faraday that electrical forces were responsible for the joining of atoms in compounds.
In 1879, Sir William Crookes studied the effects of sending an electric current through a gas in a sealed tube. The tube had electrodes at either end and a flow of electrically charged particles moved from one of the electrodes. This electrode was called the cathode, and the particles were known as cathode rays. The particles were first believed to be negatively charged atoms or molecules. However, subsequent experiments showed that these particles could penetrate thin sheets of material which would not be possible if the particles were as large as atoms or molecules.
In 1895, Wilhelm Roentgen, experimenting with cathode rays, discovered new and different kinds of rays. Roentgen discovered that if he directed these rays toward a paper plate coated with barium platinocyanide, the plate became fluorescent. During subsequent experiments, he found the rays created an image on a photographic plate. These “new” rays were originally known as Roentgen rays. We know them today as x-rays which are part of the electromagnetic spectrum.
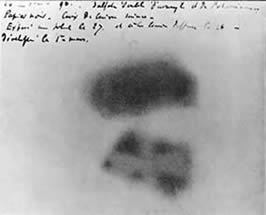
Bequerel’s photographic plate
In 1896, Henri Becquerel was studying the fluorescent properties of uranium salts and placed a piece of the uranium salt on top of a photographic plate wrapped in black paper. He discovered, upon development, that the plate was exposed in the shape of the uranium sample. Becquerel had discovered radioactivity. The radiation emitted by the uranium shared certain properties with x-rays and light. Becquerel and two of his students, Marie and Pierre Curie, shared the 1903 Nobel Prize in Physics for their studies in spontaneous radiation.
Further experiments by other scientists showed that when the beam from a radioactive ore was passed through a strong magnetic field, there were three kinds of radiation emitted. These rays were named alpha, beta, and gamma by Ernest Rutherford. Alpha radiation is a stream of positive particles composed of two protons and two neutrons (helium nuclei), beta radiation is a stream of particles with negative charges now known as electrons, and gamma radiation is part of the electromagnetic spectrum.
Based on the evidence of experiments in the latter part of the 19th century, it became apparent that the atom was not a solid sphere, and was far more complex than originally thought by the early Greek philosophers and John Dalton. A new model of the atom would have to be developed to incorporate these new findings.
In 1897, J.J. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. He demonstrated that cathode rays were negatively charged. In addition, he also studied positively charged particles in neon gas. Thomson realized that the accepted model of an atom did not account for negatively or positively charged particles. Therefore, he proposed a model of the atom which he likened to plum pudding. The negative electrons represented the raisins in the pudding and the dough contained the positive charge. Thomson’s model of the atom did explain some of the electrical properties of the atom due to the electrons, but failed to recognize the positive charges in the atom as particles.
In 1911, Ernest Rutherford, a former student of J.J. Thomson, proved Thomson’s plum pudding structure incorrect. Rutherford with the assistance of Ernest Marsden and Hans Geiger performed a series of experiments using alpha particles. Rutherford aimed alpha particles at solid substances such as gold foil and recorded the location of the alpha particle “strikes” on a fluorescent screen as they passed through the foil. To the experimenters’ amazement, although most of the alpha particles passed unaffected through the gold foil as expected, a small number of particles were deflected at an angle, and a few ricocheted straight back. Rutherford concluded that the atom consisted of a small, dense, positively charged nucleus in the center of the atom with negatively charged electrons surrounding it. The discovery of the nucleus is considered to be Rutherford’s greatest scientific work.
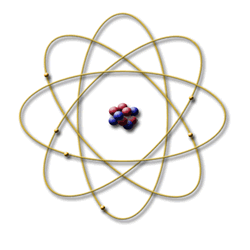
Bohr model
In 1913, Neils Bohr, a student of Rutherford’s, developed a new model of the atom. He proposed that electrons are arranged in concentric circular orbits around the nucleus. This model is patterned on the solar system and is known as the planetary model. The Bohr model can be summarized by the following four principles:

Quantum mechanical model
In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr atom model one step further. Schrödinger used mathematical equations to describe the likelihood of finding an electron in a certain position. This atomic model is known as the quantum mechanical model of the atom. Unlike the Bohr model, the quantum mechanical model does not define the exact path of an electron, but rather, predicts the odds of the location of the electron. This model can be portrayed as a nucleus surrounded by an electron cloud. Where the cloud is most dense, the probability of finding the electron is greatest, and conversely, the electron is less likely to be in a less dense area of the cloud. Thus, this model introduced the concept of sub-energy levels.
Until 1932, the atom was believed to be composed of a positively charged nucleus surrounded by negatively charged electrons. In 1932, James Chadwick bombarded beryllium atoms with alpha particles. An unknown radiation was produced. Chadwick interpreted this radiation as being composed of particles with a neutral electrical charge and the approximate mass of a proton. This particle became known as the neutron. With the discovery of the neutron, an adequate model of the atom became available to chemists.
Since 1932, through continued experimentation, many additional particles have been discovered in the atom. Also, new elements have been created by bombarding existing nuclei with various subatomic particles. The atomic theory has been further enhanced by the concept that protons and neutrons are made of even smaller units called quarks. The quarks themselves are in turn made of vibrating strings of energy. The theory of the composition of the atom continues to be an ongoing and exciting adventure.
In the fifth century BC, Democritus proposed the
concept of “atomos”, a Greek word meaning
The answer is B. The word “atomos” translates to indivisible.
Of the three major particles of the atom, which one was discovered most recently?
The answer is B. Of the three major parts of the atom (electron, proton, and neutron), the neutron was the last to be discovered by Chadwick in 1932.