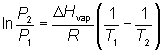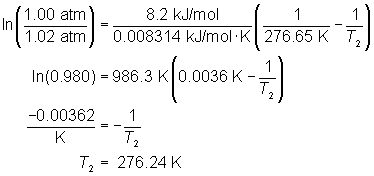username@email.com
username@email.com
In this lesson we will review the equations for internal energy, and work relevant to a system and its surroundings. We will also calculate heat flow and the energy of phase changes. Finally, we will perform calculations using the Clausius-Clapeyron equation.
When looking at equilibrium, the system is that part of the universe being studied. The surroundings describe everything else around the system being studied. The universe is defined as the system and its surroundings. Enthalpy is the internal energy of a system plus the pressure of the system multiplied by the volume of the system. Entropy can be considered as the measure of disorder or randomness of a system. The free energy of a system is the minimum amount of energy available when a system is in equilibrium with its surroundings.
The internal energy of a system is the sum of its kinetic and potential energies. It can be changed by work being done on or by the system and by heat being added or removed from the system. When considering such changes we always assume the frame of reference of the system.
ΔE = q + w
ΔE represents change in the internal energy of a system, q represents heat, and w represents work.
When 12.4 kJ of heat are added to a system on which 3.5 kJ of work is done, what is the change in the internal energy of that system?
Since ΔE = q + w, the change in the internal energy of the system, is equal to 12.4 kJ + 3.5 kJ, or 15.9 kJ.
A gas, by virtue of its compressibility, can have work done on it when the gas is compressed by an increase in pressure. Work can defined as the pressure multiplied by a change in volume, or w = PΔV. w and PΔV will have opposite signs since when a gas expands, work is done by the gas on the surroundings, and when a gas contracts work is done on the system. The equation becomes:
w = –PΔV
How much work is done when a gas expands from 23 L to 34 L at a constant pressure of 3.2 atm?
w = –PΔV
w = –(3.2 atm) (34 L – 23 L)
w = –35 atm . L
Calculate ΔE when 84.2 kJ of work are done on a system when 321 J of heat are added.
The correct answer is C. ΔE is the sum of heat and work. The units need to be the same, so the joules of heat needs to be divided by 1000 to obtain kilojoules.
ΔE = q + w
ΔE = [(321 J)/1000] + 84.2 kJ
ΔE = 84.5 kJ
Calculate the work done on a system when its volume increases from 32.3 L to 48.2 L at a constant pressure of 0.931 atm.
The correct answer is B. The change in volume is the final volume minus the initial volume and then multiplied by the pressure, not divided. Remember that:
w = –PΔV·w = –(0.931 atm)(48.2 L –32.3 L)w = –14.8 atm·L
Calorimetry is the science of measuring heat transfer from one object to another. The quantity of heat energy transferred may be calculated using the equation, Q = mCpΔT, where Q is heat, m is the mass of the substance, Cp the specific heat of the substance and ΔT is the difference in temperature (final – initial).
Calculate the final temperature of a sample of Te (Cp = 0.201 J/g°C) if 123.4 g if its initial temperature is 56.7 °C and it absorbs 8901 J of heat.
Q = mCp(Tfinal − Tinitial)
8901 J = (123.4 g)(0.201 J/g C°) (Tfinal − 56.7°C)
8901 J = (24.8034 J/°C)(Tfinal − 56.7°C)
358.9 °C = (Tfinal − 56.7°C)
Tfinal = 415.6°C
How much thermal energy is released by 123 g of H2O when its temperature decreases from 45.6°C to 7.89°C?
The specific heat of water is 4.184 J/g°C.
Q = mCp(Tfinal − Tinitial)
Q = (123 g)(4.184 J/g°C)(7.89 °C − 45.6 °C)
Q = (123 g)(4.184 J/g°C)(-37.71 C°)
Q = –19406 J
Q= –19.4 kJ
How much thermal energy is absorbed by 123.4 g H2O when its temperature increases from 56.7°C to 89.0°C?
The correct answer is C. The heat will be positive since the temperature change is positive, and one must remember to calculate the change in temperature and not use just the final temperature.
Q = mc (Tfinal − Tinitial)
Q = (123.4 g)(4.184 J/g°C)(89.0 C° − 56.7 C°)
Q = 16.7 kJ.
Calculate the specific heat of a metal if 293 grams of the metal release 282 J when it is cooled from 45.6°C to 38.2°C.
The correct answer is B. Specific heat for an object is always positive. One must remember to use the change in temperature and not just the final temperature.
Q = mCpΔT,
28200 J = (293 g)(Cp)(38.2 °C – 45.6 °C)
Solving for Cp, the specific heat of the metal is: 0.130 J/g°C
If 1234 J are absorbed by 567 g of H2O at an initial temperature of 8.90°C, what is the final temperature?
The correct answer is C. Remember the specific heat for water is 4.184 J/g°C. The final temperature will be positive:
Q = mcΔT
1234 J = (567 g)(4.184 J/g°C)(Tfinal – 8.90 °C)
Tfinal = 9.42 °C
During a phase change, the temperature of a substance does not change. The amount of heat transferred to the substance depends on the mass of the material and what it is made of. Because there is no temperature change, the heat equation becomes:
q = mΔHfus
q is heat measured in Joules, m is mass measured in grams, and ΔHfus is the enthalpy of fusion (if vaporization, it will read ΔHvap) measured in kJ/mol. This means the mass must be expressed in moles. If the value is in J/g or kJ/g the value need not be converted into moles.
To determine the amount of energy required to change a given mass of solid to a gas, four separate heat transfer calculations must be considered. They are:
q = mCpΔT for heating the solid
q = mΔHfus from the solid to liquid phase
q = mCpΔT for heating the liquid, and
q = mΔHvap for the phase change from liquid to gas
Calculate the heat needed to melt 19.28 g sodium chloride (NaCl), if its enthalpy of fusion is 30.2 kJ/mol.
q = mΔHfus
q = (0.3299 mol NaCl)(30.2 J/mol)
q = 9.96 kJ
Determine the amount of energy required to convert 193.2 g ice at –25.0°C to steam at 125°C.
The specific heat of ice is 2.06 J/g°C.
The specific heat for water is 4.184 J/g°C.
The specific heat for steam is 2.02 J/g°C.
The enthalpy of fusion for ice is 334 J/g.
The enthalpy of vaporization for water is 2260 J/g.
Heating of ice:
q = mCpΔT
q = (193.2 g)(2.06 J/g°C)(0°C– (-25.0°C))
q = 9949.8 J
Ice → water
q = mΔHfus
q = (193.2 g)(334 J/g)
q = 64528.8 J
Heating of water
q= mcΔT
q = (193.2 g)(4.184 J/g°C)(100°C– 0°C)
q = 80834.88 J
Water → steam
q = mΔH vap
q = (193.2 g)(2260 J/g)
q = 436632 J
Heating the steam
q = mCpΔT
q = (193.2 g)(2.02 J/g°C)(125°C – 100°C)
q = 9756.6 J
How much energy is needed to melt 13.57 g sodium fluoride (NaF)? The enthalpy of fusion for sodium fluoride is 29.3 kJ/mol.
The correct answer is A. Choices B and C show incorrect mole conversions, while choice D is obtained by failure to convert to moles.
q = mΔHfus
q = (0.3232 mol)(29.3 kJ/mol)
q = 9.47 kJ
How much energy is needed to change 4392 g ice at –7.5°C into steam at 103.4°C?
The specific heat of ice is 2.06 J/g°C.
The specific heat for water is 4.184 J/g°C.
The specific heat for steam is 2.02 J/g°C.
The enthalpy of fusion for ice is 334 J/g.
The enthalpy of vaporization for water is 2260 J/g.
The correct answer is C. The others reflect erroneous conversions to kJ or incorrect multiplications.
Heating of ice
q = mcΔT
q = (4392 g)(2.06 J/g °C)(0 °C– (-7.5 °C))
q = 67856.4 J
Ice → water
q = mΔHfus
q = (4392 g)(334 J/g)
q = 1466928 J
Heating of water
q = mcΔT
q = (4392 g)(4.184 J/g °C)(100 °C– 0 °C)
q = 1837612.8 J
Water → steam
q = mΔHvap
q = (4392 g)(2260 J/g)
q = 9,925,920 J
Heating the steam
q = mcΔT
q = (4392 g)( 2.02 J/g °C)(103.4 °C – 100 °C)
q = 30164.256 J
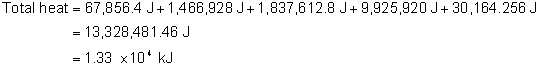
The Clausius-Clapeyron equation provides a method to determine vapor pressure or temperature using the heat of vaporization. It can also be used to obtain values for ΔHvap.
The equation is:
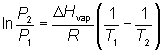
Determine the normal boiling point for a substance with a vapor pressure of 1.02 atm at a temperature of 3.5°C. The heat of vaporization for the substance, methane, is 8.2 kJ/mol.
Hint: Remember the normal boiling point occurs at 1 atm.