 username@email.com
username@email.com
In this lesson we will study the kinetic molecular theory and the basic properties of gas laws and their calculations.
The kinetic molecular theory is based on the assumption of an ideal, or “perfect,” gas. Although the perfect gas does not exist in the real world, the kinetic molecular theory is describing this imaginary gas and the KMT is extremely useful for predicting gas behavior. Remember, the KMT is a theory. It has been proven many times, but it is not absolute. It is an attempt to treat all real gases as if they were the same and their differing behaviors are approximated by the ideal gas.
The ideal gas model states:
Real gases are defined as gases that do not fit the kinetic molecular theory. Even one deviation places the gas in the real gas category. The KMT will always cause problems for scientists, after all, it is a theory and still being tested. However, two main characteristics cause deviations from ideal behavior:
When the system is at high pressure or low temperature it again deviates from ideal behavior. These circumstances cause the particles to be close together and improve the chance of interactions.
The four variables used to describe gases are temperature, pressure, moles, and volume. Temperature is the measure of the average kinetic energy of random motion of the particles in a sample of matter. Because many properties of gases depend on the temperature of the studied system, calculations with gases include a specific temperature. Kelvin is used for all gas law calculations. If you are given a value in ºC, it must be converted to Kelvin.
K = °C + 273
T = t + 273
K = Kelvin
T = absolute temperature
t = Celsius temperature
Pressureis the force exerted per unit area. In relation to gases, pressure is a measure of the total force exerted by the moving particles of a gas as they collide with the walls of the container.
| Pressure | Temperature |
|---|---|
| 1.0000 atm | 273.16 K |
| 101.325 kPa | 273.16 K |
| 760.0 mm Hg | 273.16 K |
| 760.0 torr | 273.16 K |
Moles is the fundamental SI unit used to measure the amount of a substance. Moles tell you the quantity of the gas. This expresses the number of objects in the system and does not directly indicate their masses.
Volume is the amount of space an object occupies. Gas particles are widely spaced and the volume of a gas is primarily empty space between particles. As a gas contracts, its particles move closer together. As it expands, they move farther apart.
Graham’s Law of Effusion states that a gas will effuse at a rate that is inversely proportional to the square root of its molecular mass, µ.
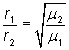
r1 = velocity of gas 1
µ1 = molecular mass of gas 1
r2 = velocity of gas 2
µ2 = molecular mass of gas 2
An O2 molecule travels at 480 m/s at room temperature. How fast would a molecule of SO3 travel at the same temperature?
First, list the given values:
r1 = 48 0 m/s
µ1 = 32 g/mol (16 g/mol × 2)
µ2 = 80 g/mol (32 g/mol + 16 g/mol × 3)
Then, write the equation:
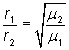
![]() ;r2 = 304 m/s
;r2 = 304 m/s
Dalton’s Law states that the sum of the individual pressures of all the gases that make up a mixture is equal to the total pressure.
PT = P1 + P2 + P3 + …, or ![]()
A 32.0 mL sample of H2 gas collected over water has a pressure of 750.0 torr. What is the partial pressure of H2 gas if the total pressure is 875.5 torr?
P1 = 750.0 torr
P2 = P 2
PT = 875.5 torr
PT = P1 + P2 +
P3 + …..
875.5 torr = 750.0 torr + P2
P2 = 125.5 torr
A 15.0 mL sample of N2 gas collected over water has a pressure of 1.00 atm. What is the partial pressure of the N2 gas if the total pressure is 4.6 atm?
P1 = 1.00 atm
P2 = P2
PT = 4.6 atm
PT = P1 + P2 +
P3 + …..
4.6 atm = 1.00 atm + P2
P2 = 3.6 atm
The correct answer is 3.6 atm.
Charles’s Law states that the volume of a gas varies directly with the Kelvin temperature, assuming that the pressure is constant. As one goes up, the other goes up.
A sample of nitrogen occupies a volume of 250.0 mL at 298 K. What volume will it occupy at 368 K?
V1 = 250 mL
T1 = 298 K
T2= 368 K
![]()
![]()
V2 = 309 mL
Boyle’s Law states that the volume of a gas varies inversely with its pressure if temperature is held constant. As one goes up, the other goes down.
P1 × V1 = P2 × V2
A sample of CO2 gas occupies a volume of 3.50 L at 125 kPa. What pressure would the gas exert if the volume were decreased to 2.0 L?
P1 = 125 kPa
V1 = 3.50 L
V2 = 2.0 L
P1 × V1 = P2 × V2
125 kPa × 3.50 L = P2 × 2.0 L
P2 = 220 kPa
Ammonia gas occupies a volume of 250.0 mL at a pressure of 720.0 mm Hg. What volume will it occupy at STP?
P1 = 720.0 mm Hg
V1 = 250.0 mL
P2 = 760.0 mm Hg
P1 × V1 = P2 × V2
720.0 mm Hg × 250.0 mL = 760.0 mm Hg × V2
The correct answer is 236.8 mL.
Ideal Gas Law uses the four gas variables in relationship to each other. Remember temperature is always calculated in Kelvin.
PV = nRT
R = 0.0821 L∙atm/mol∙K
R = 8.314 L∙kPa/mol∙K
How many moles of oxygen will occupy a volume of 2.5 L at 1.2 atm and 298 K?
P = 1.2 atm
V = 2.5 L
PV = nRT
1.2 atm × 2.5 L = n × (0.0821 L∙atm/mol∙K) × 298 K
n = 0.12 mol
Be sure to use the same pressure units for the problem and the R constant.
Calculate the volume of 1 mol of CCl4 at STP.
P = 1.0 atm
n = 1 mol
T = 273.16 K
PV = nRT
1.0 atm × V = 1 × (0.0821 L∙atm/mol∙K) × 273.16 K
V = 22.4 L.