 username@email.com
username@email.com
In this lesson we will review general science knowledge, including but not limited to mass, weight, mixtures, elements, atoms, molecules, and properties of matter. We will also distinguish between ionic and molecular compounds.
Matter is anything that has mass and occupies space. The computer you are using is matter. The cell phone that you use is matter. Matter is all around us and affects every aspect of our lives. Mass is how much matter an object has. Weight is the force of gravity pulling on a quantity of matter. For example, on Earth, Jim weighs 200 lbs. On the moon he weighs 33.3 lbs. The moon’s gravity is 1/6 of earth’s. However, Jim still has the same mass; he still occupies the same amount of space. He did not lose an arm or a leg to take up less space.
Matter is made up of elements which are made up of atoms. The building block of matter is the atom. An atom is the smallest particle that has the properties of an element. Atoms may sometimes have an electrical charge on them, + or –. Atoms with a charge, either positive or negative are called ions. Atoms combine to form molecules. Molecules are a neutral group of atoms held together by chemical bonds. Molecules cluster and form elements. Elements are the simplest substances on earth. Elements are substances that cannot be broken down into simpler substances. Elements affect human life daily, from the air we breathe (O2), to the calcium (Ca), in our bones, we need elements to survive. Just like atoms, elements combine to form compounds and mixtures.
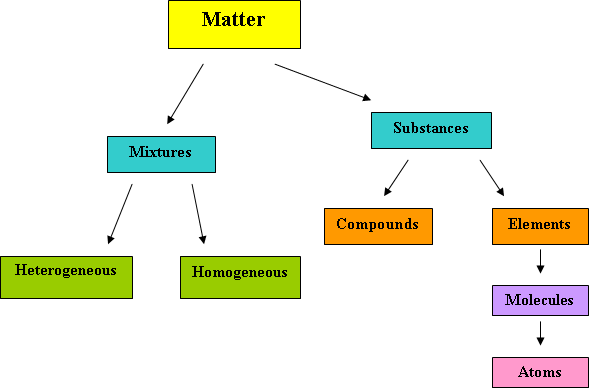
Compounds are substances made of atoms of more than one element bound together. For example, hydrogen (H2) and oxygen (O2) combine to form water. 2H2 + O2 → 2H2O. Compounds are extremely important to us. Water to drink, foods to eat, and medicines to take are only a few examples of why compounds are vital to humans. However, compounds do not retain the properties of their components.
The two main types of compounds in chemistry are ionic and molecular compounds. Ionic compounds are compounds composed of cations and anions combined to have a neutral net charge. Ionic compounds transfer electrons. Cations are positively charged particles. Anions are negatively charged particles. Molecular compounds are substances consisting of atoms that are covalently bonded. Covalent bonds are formed when atoms share pairs of electrons.
Mixtures are a combination of more than one pure substance. A pure substance is any matter that has a fixed composition and definite properties. For example, when you purchase pure orange juice from the grocery store, this implies that you are only getting the juice of the orange and no other additives. Another example of a pure substance is water; it is only hydrogen and oxygen combined in definite proportions. Combining pure substances gives us mixtures. Unlike compounds, mixtures do retain the properties of their components. For example, chocolate milk is a mixture. You can still taste the milk and chocolate in the mixture.
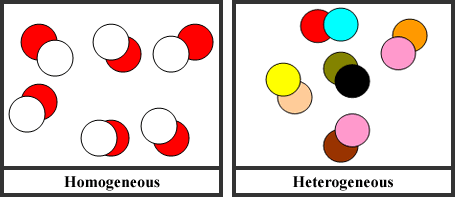
There are two types of mixtures: heterogeneous and homogeneous. Heterogeneous mixtures are mixtures that are not uniformly distributed. Heterogeneous mixtures can have different properties throughout the mixture. An example would be a salad. There are many different things in any salad: lettuce, carrots, mushrooms, peppers, cheese, tomatoes, and more. You can see each portion of the different ingredients in the salad, which makes it a heterogeneous mixture. Homogeneous mixtures are uniformly distributed. Each portion of a homogeneous mixture, no matter how small or large will have the same composition throughout. Sodas are an example of homogeneous mixtures that many people drink daily. Soda contains sugar, syrup, and carbonic acid, to name some ingredients, yet we do not see each individual ingredient in the soda, they are all mixed together uniformly.
All matter has properties. Properties are characteristics of a substance. For matter there are two main types of properties: intensive and extensive. Intensive properties are physical properties of a system that do not depend on the system’s size or the amount of material in the system. Types of intensive properties include color, density, texture, shape, melting point, and more. Extensive properties are physical properties that do depend on the system size and amount of material in the system. Examples of extensive properties are viscosity, solubility, volume, mass, and energy.
There are three main states of matter: solid, liquid, and gas. Solids have a rigid volume and vibrate about a fixed position. Liquids flow more freely than solids, and their volume will assume the shape of the container they are place into. Gases are very free flowing and have the most energy of the three states. Gas molecules are generally too far apart to significantly interact with each other.

Phase diagrams are graphic representations of the relationships between the physical states (solid, liquid, and gas) of a substance. Phase diagrams depict the effects of pressure and temperature on the three main states of matter.
Water, for example, is a pure substance because it has a fixed composition, H2O. An increase of pressure on water will cause it to change from a gas state (steam or water vapor) to liquid then to a solid state (ice). Also, increasing the temperature will cause the solid (ice) to melt to liquid. If heating is continued, the liquid water will then change into water vapor. Water vapor is the gas released when water is boiling.
Phase diagrams can also show normal boiling point, normal freezing/melting point, triple point and the critical point. The normal boiling point is the temperature at which a substance boils at 1.0000 atm of pressure. The normal freezing/melting point is the temperature at which a substance melts and freezes at 1.0000 atm of pressure. The triple point is the temperature at which all three states of a substance exist in equilibrium (balance). The critical point is at the end of the reaction.
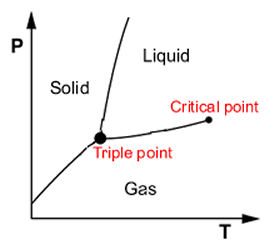
Phase Diagram
Classify the following substances as homogeneous or heterogeneous mixtures:
chocolate milk – homogeneous
pepperoni pizza – heterogeneous
taco salad – heterogeneous