 username@email.com
username@email.com
We will review how atomic orbitals may be combined within an atom to form hybrid orbitals, or combined between two atoms to form molecular orbitals.
You may remember that we often describe electrons in an atom by the orbital in which they reside. Nitrogen for example, has two electrons in the 1s orbital, two electrons in its 2s orbital and three electrons spread out among its three 2p orbitals. We often show this in the form of an electron configuration, which for nitrogen would look like this: 1s2 2s2 2p3. These orbitals are derived for individual atoms and thus may be referred to more specifically as atomic orbitals.
It is important to remember that atomic orbitals are not physical objects, but rather they are simply regions of space defined relative to the nucleus of the atom. When atoms bond covalently, they must share electrons. Since these shared electrons must continue to belong to their original atoms, they must remain in their original orbitals. But since they must now also belong to a new atom, they must also now reside in an orbital for that atom as well. The only solution to this problem is that an orbital of one atom must overlap with an orbital of another atom in order to form a covalent bond.
The reason for this background is to remind us that atomic orbitals are useful in describing electron behavior in atoms. They are less useful when those atoms begin to bond with each other to form molecules. For example, we know that carbon will bond with hydrogen to form methane, CH4. In order for these bonds to form, the orbitals containing carbon’s valence electrons (2s and 2p) must overlap with each hydrogen’s valence orbital (1s). The molecule formed is predicted to be tetrahedral in shape, with bond angles of approximately 109.5º. But p orbitals are 90º apart from each other and the s orbital is superimposed upon those p orbitals.
We can resolve this contradiction by the use of hybrid orbitals. Since atomic orbitals are simply mathematical equations that successfully describe electron behavior, we can combine the equations to get new orbitals. By combining the 2s and the three 2p orbitals for carbon, we get four new orbitals which we will call sp3 orbitals. Fortunately, these four orbitals are arranged in a tetrahedral pattern. In the figure below, we see the 2s orbitals and then the three 2p orbitals. On the right we see, the four sp3 hybrid orbitals superimposed upon one another on the right.


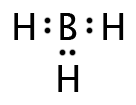
There are three hybridization schemes or types of hybrid orbitals that can form when atoms obey the Octet Rule. It is easiest to recognize them from a Lewis structure of the molecule. Just as we did in VSEPR, we will look at the number of regions of electron density around a central atom. Remember that double bonds or triple bonds still count as only one region.
We have already seen that four regions of electron density, like that of CH4, will cause the 2s and the three 2p orbitals to hybridize and form four sp3 hybrids. This is true even if any of the regions are lone pairs, therefore molecules such as H2O and NH3 are also sp3 hybridized.

When a molecule has three regions of electron density around a central atom, such as C2H4, the s orbital and only two p orbitals will combine to form three sp2 hybrid orbitals. These new orbitals are 120º apart, which is the same as predicted by VSEPR. The figure shows the three sp2 hybrid orbitals superimposed upon one another.

A molecule with only two regions of electron density around a central atom, such as C2H2, will combine its s orbital with only one p orbital, forming two sp hybrid orbitals. These orbitals are 180º apart, so they form linear molecules as predicted by VSEPR. The figure below shows the two sp hybrid orbitals superimposed upon one another.
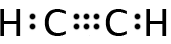

Which of the following is the correct hybridization for the nitrogen atom in methylamine, CH3NH2?
The correct answer is C. In the Lewis structure of CH3NH2, nitrogen has four regions of electron density (a single bond to the carbon atom, two single bonds to hydrogen atoms and a lone pair of electrons). This is consistent with sp3 hybridization.
You may recall that in order for an atom to have more than eight valence electrons, it must use one or more d orbitals. If we combine it (or them) with the s and p orbitals, we will make hybrid orbitals that correspond to the shapes that we saw previously when discussing VSEPR.
Five regions of electron density will result in dsp3 hybrid orbitals. These orbitals are arranged in exactly the same fashion as the trigonal bipyramidal shape—three are equatorial and 120º from each other in a triangular plane while two are axial, each perpendicular to the plane of the equatorial orbitals, one above and one below. The figure below shows the five dsp3 hybrid orbitals superimposed upon one another.


Which of the following is the correct hybridization for the sulfur atom in sulfur tetrachloride, SCl4?
The correct answer is B. In the Lewis structure of SCl4, sulfur has five regions of electron density (four single bonds to chlorine atoms and a lone pair of electrons). This is consistent with dsp3 hybridization.
As we just saw in hybrid orbital theory, atomic orbitals in a given atom can combine with each other to make new orbitals. These new hybrid orbitals then overlap with other orbitals to form covalent bonds. Another way to compensate for the inability of atomic orbitals to describe electrons in a molecule is to use molecular orbital theory. In this theory, the atomic orbitals from one atom combine with the atomic orbitals of another atom to form molecular orbitals.
Molecular orbital theory can be quite complex, so we will only consider a few simple aspects of it here. One important point is that any time orbitals are combined, the number of orbitals is conserved. You may recall that when we combined an s orbital with a single p orbital, we formed two sp hybrid orbitals. Since we started with two orbitals, we ended with two orbitals. This will also apply to molecular orbitals. If we combine two 1s orbitals from two different atoms, we will form two new molecular orbitals. These are traditionally called the 1σ orbital and the 1σ* orbital.
The 1σ orbital is lower in energy than either of the 1s orbitals used in its making. It has its greatest electron density between the nuclei of the atoms. Filling this orbital with two electrons contributes the possibility of forming a bond between the atoms, so it is called a bonding orbital.
The 1σ* orbital is higher in energy than either of the 1s orbitals used in its making. It has two regions of electron density, each on the side of one nucleus opposite the other nucleus. Putting electrons in these positions destabilizes a bond, so this type of orbital is called an antibonding orbital. A picture of two 1s orbitals combining to form the 1σ and 1σ* orbitals is shown below.

Bond order can be determined by assuming that a pair of electrons in a bonding orbital creates a bond and a pair of electrons in an antibonding orbital destroys a bond. The formula for determining bond order is as follows:

What is the hybridization of xenon in xenon difluoride, XeF2?
The correct answer is C. The Lewis structure of XeF2 has five regions of electron density around the xenon, consistent with dsp3 hybridization.
What is the hybridization of xenon in xenon tetrafluoride, XeF4?
The correct answer is D. The Lewis structure XeF2 has six regions of electron density around the xenon, consistent with d2sp3 hybridization.
