 username@email.com
username@email.com
In this lesson we introduce some basic concepts of organic chemistry.
The study of compounds containing carbon atoms is known as Organic Chemistry. There are a few notable exceptions such as the carbonates. Of the approximately 2 million known compounds one and a half million are classified as organic. The remaining are inorganic compounds. The reason for the large number of compounds is carbon’s ability to form long chain polymers.
The carbon atom, having four valence (outer shell) electrons, needs to have four bonds to be stable. The typical structure for a hydrocarbon (a molecule containing carbon and hydrogen) is:
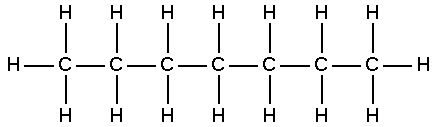
CH3CH2CH2CH2CH2CH2CH3 or CH3(CH2)5CH3
However, since it is the carbon atom that is the important part of the structure, frequently the same structure is written without the hydrogen atoms.
In the molecule C-C-C-C-C-C-C the 16 hydrogen atoms are assumed. When there are only single bonds between the carbon atoms the formula for finding the number of hydrogen atoms is CxH2x+2. Since the carbon atom needs four bonds, each carbon would need two more bonds one going up and one going down plus the two end carbons would need one additional bond going out to the side.
Another abbreviation is known as a line structure where not only the hydrogen atoms are assumed, but the carbon atoms are as well. There is a carbon atom at each end of each line and/or at each bend. The same molecule written as a line structure would be:
a) What would be the condensed formula for C-C-C-C-C?
b) What would its line structure be?
c) How many hydrogen atoms are assumed in C-C-C-C-C?
a) CH3CH2CH2CH2CH3 or CH3(CH2)3CH3 because each carbon atom has four bonds and it is assumed that if a bond is missing there is an attached hydrogen.
b)![]()
A carbon atom is assumed at each end and at each bend. This would represent the five carbon atoms.
c) 12. Each end carbon has three assumed hydrogen atoms and the three middle carbons have two assumed hydrogen atoms. Each carbon will have four atoms attached to it, the end carbons already have one carbon atom attached therefore 3 hydrogen atoms are assumed. Each middle carbon has two carbons attached to it, one on each side so each middle carbon will have two assumed hydrogen atoms.
The naming system for organic molecules is based on the number of carbon atoms and the type of bonds it contains. The following numbering system for prefixes is used to identify the number of carbon atoms:
Hydrocarbons containing only single bonds are classified as saturated. Hydrocarbons containing one or more carbon-carbon multiple bonds are classified as unsaturated.
Saturated hydrocarbons are also classified as alkanes. In naming straight chain or unbranched hydrocarbons the name is just the number of carbon atoms using the above organic prefix and using -ane as the ending to indicate the molecule has only single bonds.
C3H8 or C-C-C is propane. Prop- due to the three carbons and the – ane for having only single bonds.
H8C18or C-C-C-C-C-C-C-C is octane.
Naming branched alkanes follows a systematic naming system where the longest continuous chain becomes the last name and the branches are like charms on a charm bracelet. The charms or hydrocarbon branches are referred to as alkyl groups. They are named by using the organic root number but instead of ending in -ane, they end in -yl.
A two carbon branch is called an ethyl group and three carbon branch is a propyl group, etc.
In addition, we need to list the location of the branch. If we have more than one of the same type of branch we need to say how many we have using the following:
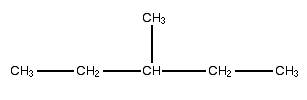
In this molecule, the longest continuous chain is straight across and contains 5 carbons so the last name is pentane. However, it has a single one-carbon branch, so the name is methyl pentane. Since the methyl group is on the 3rd carbon over it is 3-methyl pentane.
To determine the number location of the branch, number the longest chain in both directions and choose the lower number.
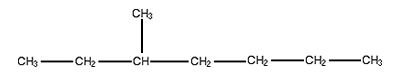
The last name of this molecule is heptane since the longest chain is straight across and contains 7 singly bonded carbon atoms. It has one carbon branch, so it we have a methyl heptane. When numbering from left to right the branch is attached to the 3rd carbon and when numbering from right to left it is attached to the 5th carbon. Since 3 is smaller than 5 the name is 3-methyl heptane.
How many carbon atoms are in this molecule?
The correct answer is B. There is a carbon at each end and each bend.
How many hydrogen atoms are attached to this molecule?
The correct answer is C.There are four carbon atoms — one at each end and one at each bend. The end carbons each have three assumed hydrogen atoms and the two middle carbons each have two assumed hydrogen atoms. 3 + 3 + 2 + 2 = 10
What is the name of the following molecule?
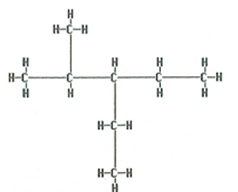
The correct answer is A. There are two branches attached to a five-carbon singly bonded main chain hence the ending of pentane. There is a two-carbon branch (ethyl) and a one-carbon branch (methyl). The branches are listed in alphabetical order (ethyl-methyl). Also, the main chain is numbered in both directions left to right and right to left. The numbers used would be the smaller set of numbers. In this case numbering from right to left the branches would be on the number three and the number four carbon. If numbered from left to right the branches are attached to the number two and number three carbons. 2 + 3 = 5 and 3 + 4 = 7 since five is smaller than seven the combination of two and three are used.