 username@email.com
username@email.com
In this lesson we will discuss chemical kinetics, which is the study of rates of reactions.
Rate is a term used frequently in physical science, especially in physics, where rate of displacement or velocity and rate of change of velocity or acceleration are well recognized. The term kinetics is used to describe the study of rates of chemical reactions.
The term “rate” implies how one quantity changes with respect to time. In velocity, it is a displacement in meters or kilometers per unit time of seconds or hours. In acceleration, the velocity changes per unit time, having units of meter/second/second. In chemical reactions the quantity which changes per unit time is the concentration of a reactant or product. The concentration of a reactant will decrease as it is consumed over time; the concentration of a product will increase as it is produced over time. The units of rates of reaction are therefore moles/liter/second or M/s, where M represents moles per liter. Brackets, such as [Cl], are sometimes used to show units of molar concentration.
The diagram below shows the decomposition reaction of gaseous N2O5 into its elements as represented by the equation
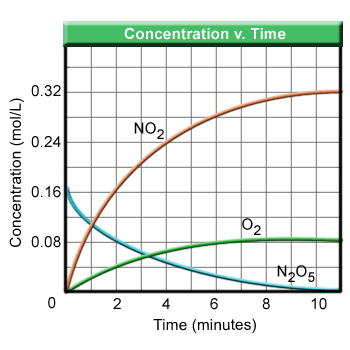
From the slope of the reactant line on the diagram, one can see that the rate of change of the reactant is not constant. The slope is steepest at the beginning of the reaction and then levels off. The rate is fastest when the concentration of the reactant is the highest. The rate of the reaction can be defined then as the change in molar concentration of reactant [R] per unit time. Or 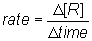 .
.
However, the concentration is decreasing and rates are always considered positive, so a negative sign is used to show that the concentration of the reactant has decreased.
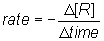
Thus in the reaction of ![]() , the rate of disappearance of Y2 would be one-half the rate of disappearance of X2. The rate of formation of product would be equal to the rate of disappearance of X2, but twice that of the rate of disappearance of Y2. A quantitative expression called the rate equation can be written to show that the rate is proportional to the concentration of the reactants and products.
, the rate of disappearance of Y2 would be one-half the rate of disappearance of X2. The rate of formation of product would be equal to the rate of disappearance of X2, but twice that of the rate of disappearance of Y2. A quantitative expression called the rate equation can be written to show that the rate is proportional to the concentration of the reactants and products.
There are two types of rate laws:
Consider the example showing the decomposition of N2O5. Imagine the following rate data was obtained for different initial concentrations of N2O5.
|
Initial Concentration N2O5 (mol/L)
|
Initial Rate of Reaction (mol/L/s)
|
|
0.84 M
|
3.6 × 10-4
|
|
0.42 M
|
1.8 × 10-4
|
These results show that when the concentration of N2O5 is halved, the rate is also halved. This means that the reaction is first order with respect to the N2O5 and the rate equation can be written as
![]() or simply
or simply ![]()
Let’s look at an example of how to determine the rate equation when given the experimental data.
The following initial rates of reaction were measured for the concentrations of reactants shown in the table for the reaction of ![]() . Determine the rate equation.
. Determine the rate equation.
|
Experiment number
|
Initial Conc. A
|
Initial Conc. B
|
Rate, mol/L/s
|
|
1
|
0.100 M
|
0.005 M
|
1.35 × 10-7
|
|
2
|
0.100 M
|
0.010 M
|
2.70 × 10-7
|
|
3
|
0.200 M
|
0.010 M
|
1.08 × 10-6
|
The general form of the equation would be ![]() . The data will be used to determine the value of the exponents. Considering experiments 1 and 2, you can see that keeping the concentration of A constant, and doubling the concentration of B, causes the rate to increase by a factor of two (the same as the increase in the concentration of B). Thus, the order of the reaction with respect to B is first order and the value of m would be 1.
. The data will be used to determine the value of the exponents. Considering experiments 1 and 2, you can see that keeping the concentration of A constant, and doubling the concentration of B, causes the rate to increase by a factor of two (the same as the increase in the concentration of B). Thus, the order of the reaction with respect to B is first order and the value of m would be 1.
If you consider the experiments 2 and 3 where the concentration of B is held constant, and the concentration of A is doubled, the rate is increased by a factor of 4;![]() . This means that the rate depends on the square of the concentration of A, and the exponent n has a value of 2.
. This means that the rate depends on the square of the concentration of A, and the exponent n has a value of 2.
The rate equation can be written as ![]() . The reaction is second order with respect to reactant A and first order with respect to reactant B; the overall order of the reaction is third order which is the sum of the exponents.
. The reaction is second order with respect to reactant A and first order with respect to reactant B; the overall order of the reaction is third order which is the sum of the exponents.
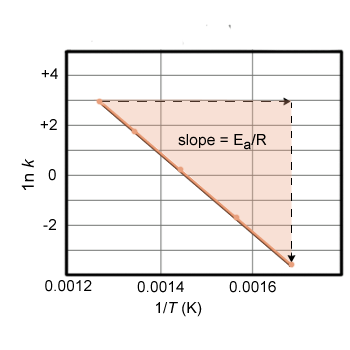
We can also use this data to determine the value of the rate constant k. We can take the experimental data for any of the experiments and substitute it into the rate equation. In this case:
k = 0.0027 L2/mol2s
To use the integrated form of the rate equation, one must plot the concentration of the reactant of interest as a function of time. Unless the reaction is zero-order, the graph will be a curve. The rate of the reaction can be determined by the slope of the line, but to determine the rate equation more calculations must be done. Using calculus, it can be shown that the equation relating the concentration as a function of time for a first order reaction is:
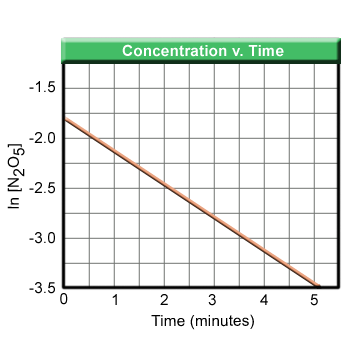
The half-life of any reaction is the time it takes for the concentration of the reactant to become 50% of the original amount. In other words, the ratio of [A] to [A]0 is equal to 0.50.
Substituting the values of [A] = 0.5[A]0 in the integrated rate equation, ![]() , and rearranging gives:
, and rearranging gives:
![]() where t = the half-life, t½.
where t = the half-life, t½.
Because ln0.50 = 0.693, for any first order reaction< ![]() ; the half-life is independent of the concentration of starting material.
; the half-life is independent of the concentration of starting material.
For second order reactions, the integrated rate law takes the form of ![]() . A plot of
. A plot of ![]() as a function of time will produce a straight-line with a slope equal to k. Calculations show that the equation for the half-life is
as a function of time will produce a straight-line with a slope equal to k. Calculations show that the equation for the half-life is ![]() .
.
The table below shows the characteristics of three different orders of reactions for the integrated form.
|
Order
|
Rate expression
|
Equation
|
Half-life
|
Linear plot
|
|
0
|
Rate = k
|
|
|
[A] vs. t
|
|
1
|
Rate =k[A]
|
|
|
ln[A] vs t
|
|
2
|
Rate = k[A]2
|
|
|
t |
The following data were obtained for the decomposition of gaseous HI. Determine the order of the reaction, the rate equation, the value of k, and the half-life of the reaction.
|
Time (hr)
|
0
|
2
|
4
|
6
|
|
[HI]
|
1.00
|
0.50
|
0.33
|
0.25
|
Three curves must be plotted according to the information in the summary chart above. The plot that produces a straight line will show the order of the reaction.
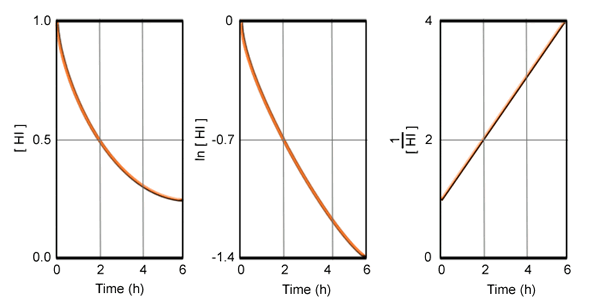
The value of k is equal to the slope of the line:
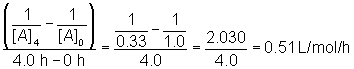
In order for a reaction to occur, the reactant particles must collide with the proper energy and proper orientation for the formation of the transition state complex. The minimum energy required is called the activation energy, Ea. There are three factors which affect the rate constant:
The Arrhenius equation relates the factors to the rate constant ![]() . The steric factor and frequency factor are combined in the variable A. A useful form of the equation is obtained by taking the natural logarithm of both sides, which yields an equation in the form y = mx + b, which can be graphed as a straight line:
. The steric factor and frequency factor are combined in the variable A. A useful form of the equation is obtained by taking the natural logarithm of both sides, which yields an equation in the form y = mx + b, which can be graphed as a straight line:
The Collision Theory assumes that in order for a reaction to occur the reacting particles must collide with the proper orientation and proper energy. Any factor that enhances the ability of the particles to interact will necessarily affect the rate. The five factors normally considered are:
As we have seen, temperature affects the rate of a reaction. According to the kinetic molecular theory, molecules are in constant motion. The higher the temperature, the higher the kinetic energy due to an increase in velocity of the molecules. In order for a reaction to occur, the reactant particles must collide with sufficient energy and the proper orientation for the formation of the transition state complex. Increasing the temperature increases both the number of collisions and the energy of collisions. An approximate rule of thumb (which is derived using the Arrhenius equation) is that an increase of 10o C doubles the rate of a reaction. For example, if the rate of a reaction at 0o C is 5.0 mol/L-min, we can expect that increasing the temperature to 10o C would make the rate 10 mol/L-min.
The concentration of a reactant is the relative amount of reactant per unit volume. Remember, the more collisions that occur, the greater the chance that a reaction will have the proper orientation. Thus, higher concentrations of reactant molecules result in a greater rate of collisions between reactant molecules, and a faster rate of reaction. Just how much the rate is increased for a particular reaction must be determined experimentally.
The nature of the substances which are reacting does affect the rate. In general, ionic reactions occur faster than molecular ones in solution. The latter require the breaking of covalent bonds and the formation of new ones. For example, a weak acid such as acetic acid reacts much more slowly with zinc metal than does the strong acid, like hydrochloric acid, of the same concentration. The difference is in the ability of the hydrochloric acid to ionize more completely than the acetic acid. Another example would be the different reaction rates of metals when they react with dilute hydrochloric acid. Magnesium metal reacts much more rapidly with acids than does lead.
The ability of reactants to meet affects the rate of a reaction. One way to enhance the opportunity for the reactants to come into contact with each other is to increase their surface area. Consider attempting to burn a charcoal briquette using the flame from a candle. The rate of combustion is very slow, because only the charcoal particles on the outside surface of the briquette are in contact with the oxygen in the air. If the briquette is ground up and blown into the candle flame the reaction rate is very rapid, in fact it is explosive. Grain elevator explosions are another impressive example of such surface area phenomena. These explosions occur, not in full grain elevators, but in ones that are almost empty of grain, but full of grain dust. A spark from a light switch can provide enough energy to start a reaction which can bring down the elevator.
A catalyst is a substance which alters the rate of a chemical reaction without itself being consumed. Since the activation energy is the minimum amount of energy required for a reaction to occur, if the particles do not have that minimum energy, no reaction occurs. Catalysts provide a different reaction pathway with a lower activation energy. Frequently the reaction mechanism for catalyzed reactions has multiple steps, each with a lower activation energy than the uncatalyzed reaction.
Catalysts may be homogeneous (same phase) or heterogeneous(different phase) with the reactants. Enzymes are examples of biological catalysts which allow reactions in the body to occur at much lower temperatures than would be required in a test tube.
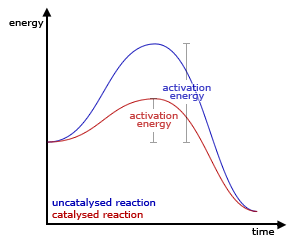
Effect of a catalyst on a reaction
Other substances called inhibitors also alter the rate of reactions by slowing them. For example, some preservatives that are found in commercial food products are inhibitors. Anti-depressant medications selectively inhibit chemical reactions in the body. The action of inhibitors is complex, but inhibitors work by reducing the effectiveness of a catalyst. It is generally thought that they offer alternate reaction pathways that result in other products.
A reaction mechanism is a proposed series of steps by which a reaction is thought to proceed. Each step in a mechanism is considered as an elementary step which may involve one particle (a unimolecular step), two particles (a bimolecular step), or in rare cases, three particles (a termolecular step). These steps have corresponding rate equations as shown below, in which the rate equation is equal to the constant multiplied by the molar concentration of each reactant.
A → B + C with rate = k[A]
A + B → C + D with rate = k[A][B]
A + B + C → D + E with rate = k[A][B][C]
When a mechanism is proposed, two requirements must be satisfied:
The slowest step in a mechanism is called the rate-determining step. The overall reaction can only proceed as fast as the slowest step. As an example, consider the formation of a compound by the reaction of nitrogen dioxide with fluorine.
![]() with rate = k[NO2][F2] determined by experiment.
with rate = k[NO2][F2] determined by experiment.
A proposed mechanism is ![]() slow
slow
With a second step of ![]() fast
fast
The first requirement is met because the algebraic sum of the two steps is the net reaction. The second requirement is also met because the rate of the overall reaction is that of the rate-determining step which is bimolecular with rate = k1[NO2][F2]. The proposed mechanism is acceptable because it meets both requirements, but it is not necessarily the correct mechanism. Chemists need to perform many additional experiments to decide the most probable mechanisms.
The mechanism for the reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and carbon dioxide is shown below. What is the overall balanced equation and what is the expected rate law?
NO2 + NO2 → NO3+ NO slow
NO3 + CO → NO2+ CO2 fast
The overall balanced equation will be the algebraic sum of the two elementary steps which is
NO3 + CO → NO2+ CO2
The rate law would be for the slowest step which is second order in NO2 and zero order in CO, so it would be rate = k1[NO2]2
A certain compound decomposes in air. The rate constant for the first order reaction is 0.423 h-1.
What is the half-life of the compound?
If a 0.050 M sample is opened, what will be the concentration after 8.0 hours?
How long will it take for the concentration to drop to 0.0030M?
For first order kinetics the integrated rate equation takes the form![]() . At the half life the concentration [A] = 0.50[Ao]. Substituting in the equation, a general form for all first order reactions is
. At the half life the concentration [A] = 0.50[Ao]. Substituting in the equation, a general form for all first order reactions is ![]() . In this case, the half-life is 1.6 hours. Using the same integrated rate equation, one can substitute in the values for initial concentration of 0.050 M, a time of 8.0 hours for t, and determine the concentration of the substance to be 0.0017 M after 8.0 hours. To determine how long it takes for the concentration to drop to 0.0030 M, the equation is solved for time and the values of k and the initial concentration are substituted. It requires 6.6 hours for the concentration to get to 0.0030 M.
. In this case, the half-life is 1.6 hours. Using the same integrated rate equation, one can substitute in the values for initial concentration of 0.050 M, a time of 8.0 hours for t, and determine the concentration of the substance to be 0.0017 M after 8.0 hours. To determine how long it takes for the concentration to drop to 0.0030 M, the equation is solved for time and the values of k and the initial concentration are substituted. It requires 6.6 hours for the concentration to get to 0.0030 M.
The decomposition of HX has an activation energy of 192 kJ/mol. The rate constant at 900°C is 0.0250 L/mol/h.
What is the rate constant at 800°C?
And at what temperature will the rate constant be 0.00625 L/mol/h?
The Arrhenius equation can be expressed in the form of the Clausius-Clapeyron equation as
![]() Subtracting the equations to eliminate the value of A, gives
Subtracting the equations to eliminate the value of A, gives
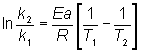 The value for R is 8.31 J/mol K. The Celsius temperatures must be converted to kelvins, and the activation energy converted to joules from kilojoules. Substituting in the equation
The value for R is 8.31 J/mol K. The Celsius temperatures must be converted to kelvins, and the activation energy converted to joules from kilojoules. Substituting in the equation
![]() Solving for k2, gives a value of 3.99 × 10-3 L/mol/h at 800°C. To determine the temperature at which the rate constant is 0.00625 L/mol/h, the same equation is used and solved for T2.
Solving for k2, gives a value of 3.99 × 10-3 L/mol/h at 800°C. To determine the temperature at which the rate constant is 0.00625 L/mol/h, the same equation is used and solved for T2.
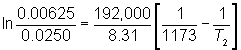 The temperature is 1262 K or 989°C.
The temperature is 1262 K or 989°C.
The following data were obtained for the reaction shown below at various temperatures. Plot the data (lnk vs 1/T) and calculate the activation energy.
|
k (s-1)
|
0.048
|
2.3
|
49
|
590
|
|
T (oC)
|
500
|
600
|
700
|
800
|
Plotting the lnk as a function of 1/T gives a straight line. The activation energy is equal to –R·(slope of the line). In this case the slope is -2.6 × 104. So calculating the activation energy is (8.31 J/mol K)(2.6 104) = 216 kJ/mol
The reaction ![]() >is second order in NO and first order in bromine gas. The rate of reaction is 1.6×10-8 mol/L/min when [NO] = 0.030 M and [Br2] = 0.020 M.
>is second order in NO and first order in bromine gas. The rate of reaction is 1.6×10-8 mol/L/min when [NO] = 0.030 M and [Br2] = 0.020 M.
What is the value of k?
The correct answer is D. It is correct since the form of the equation is rate = k[NO]2[Br2].