 username@email.com
username@email.com
We will review drawing Lewis structures for molecules and ions, including those that follow the Octet Rule and those that do not.
Lewis structures are a useful tool in determining the arrangement of atoms and types of bonds in a molecule. A typical Lewis structure represents an atom’s nucleus and its inner electrons with the element symbol, and the atom’s valence electrons with dots. It is helpful to remember that a Lewis structure is only a two-dimensional representation; therefore, it is not meant to show the three-dimensional shape of a molecule (although we will see how to infer that shape in a later lesson). It also behooves us to note that Lewis structures are not particularly useful for substances that do not form discrete molecules, such as ionic compounds, polymers or metals.
The following is a simple set of steps that we follow in drawing Lewis structures.
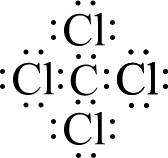
Let’s start with a simple molecule whose complete Lewis structure we saw in a previous lesson: CCl4. Carbon has four valence electrons and chlorine has seven, so the valence electrons available are: 4 + (4×7) = 32 electrons. In part (a) of the figure below, the skeleton structure is shown. Carbon has been placed in the center to make the molecule symmetrical, and there is a single bond from carbon to each chlorine atom. This uses up eight of the thirty-two electrons, so there are twenty-four electrons left. If we place six additional electrons (in pairs) around each chlorine atom, then all of our electrons have been used and each atom has eight valence electrons around it, completing an octet for each atom. This is shown in part (b).
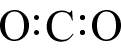 |
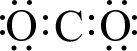 |
 |
 |
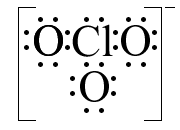
It is time to look at a polyatomic ion: ClO31−. Chlorine has seven valence electrons and each oxygen six, but we must also account for the particle’s charge. A negative charge is created when an electron is added to a neutral species, so we need to add an additional electron for the 1− charge. Our electron count looks like this: 7 + (3×6) + 1 =26 electrons. The skeleton structure has chlorine centrally located for symmetry, as shown in part (a) below. Placing six additional electrons around each oxygen provides an octet for them, as shown in part (b). At this point, we have used up twenty-four electrons, so there are two electrons left. Since the chlorine is still two electrons shy of eight, we can put the remaining electrons on the chlorine, creating a lone pair. Lone pairs will become important when we discuss shapes later. The only other thing we must do to our structure is show the charge; after all, the number of electrons is different for ClO3 vs. ClO31−. The final structure is shown in part (c).
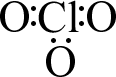 |
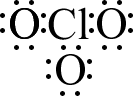 |
 |
Sometimes, a single Lewis structure may be misleading. It may imply that one bond is different from another (single vs. double, for example) when in reality they are the same. This is a situation in which we use resonance structures
Let’s consider the carbonate ion: CO32−. The electron count is as follows: 4 + (3×6) + 2 = 24 electrons. Working through the steps from above should result in any one of the structures in the figure below. Each of these structures is considered valid, in that it uses exactly 24 electrons, obeys the Octet Rule for every atom and shows the appropriate charge. The problem with any single structure is that it implies that one of the carbon-oxygen bonds is different from the other two—double vs. single. If this were correct, then experiments such as X-ray crystallography would show the double bond to be measurably shorter than the two single bonds. Instead, experimental results indicate that all three C-O bonds are of equal length, shorter than a single bond yet longer than a double bond. In other words, the “real” structure is not any one of those shown in the figure, but an average of them all.
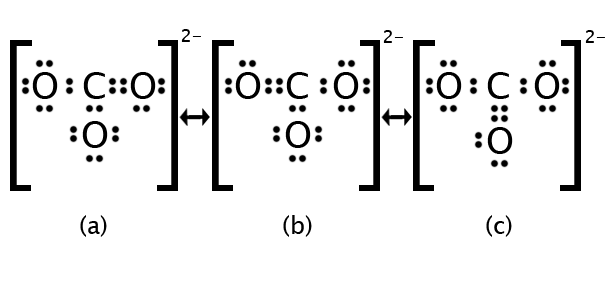
The Octet Rule is a marvelous rule of thumb, but it is far from set in stone. For example, hydrogen cannot have more than two electrons due to the lack of a p sublevel in its highest occupied energy level, n = 1. Nonmetals in the second period (B, C, N, O and F) tend to follow the Octet Rule rather rigidly, but nonmetals in the third period and beyond are capable of having more than eight valence electrons in a stable molecular structure. This is usually attributed to the presence of empty d orbitals in the highest occupied energy level, which are capable of accepting electrons in a coordinated covalent bond. In general, extra electrons (those above eight) are found on the central atom.
Let’s consider PCl5. Our electron count is: 5 + (5×7) = 40 electrons. We should recognize that the Octet Rule is a problem as soon as we draw the skeleton structure. If we connect each Cl to the P with a single bond (which would make the molecule symmetrical), then P already has ten electrons, not eight. This is shown in part (a) of the figure below. Arranging the remaining electrons around P finishes the structure, which is shown in part (b).
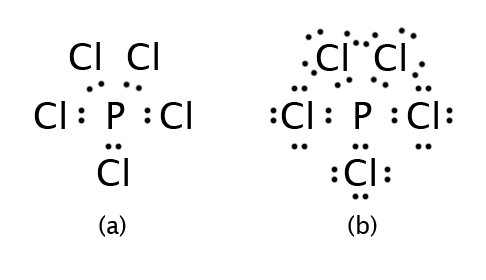
Which of the following describes the Lewis structure of the NO21− ion?
Choice B is correct. The electron count is 5 + (2×6) + 1 = 18 electrons. Choice A leaves the nitrogen electron-deficient with only six electrons. Choice C only uses 16 electrons. Choice D is incorrect because neither nitrogen nor oxygen have empty d orbitals so they must obey the Octet Rule.