 username@email.com
username@email.com
In this lesson, we will study how to name the six groups of oxygen-containing organic molecules.
We will review six groups of organic molecules that contain oxygen:
In addition to the six listed, the following are also considered oxygen containing organic compounds: epoxys (cyclic ethers), peroxides, peroxyacids, and acid anhydrides.
Alcohols contain a hydroxyl group (-OH) attached to a carbon chain.
To name an alcohol, the final “-e” of the carbon chain name is dropped and replaced with “-ol.” If the OH groups can be placed on different numbered carbons, the location of the -OH group must be included.
For example:
| CH3OH | methanol (known as wood alcohol) |
| CH3CH2OH | ethanol (the type of alcohol people drink) |
| Both of the molecules above do not need a location number for the OH group as it will be attached to the number one carbon. In ethanol either carbon would be the number one carbon in order to use the lowest number. |
|
| CH3CH2CH2OH | 1-propanol |
| CH3CH2CH2CH2OH | 1-butanol |
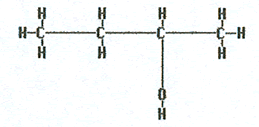 |
2-butanol |
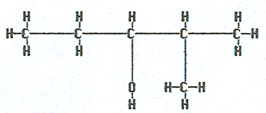 |
2-methyl-3-pentanol |
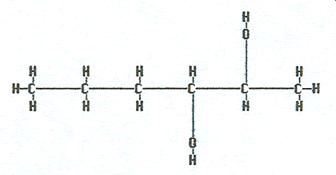 |
2,3-hexanediol |
Ethers contain an oxygen atom between two carbon atoms. There are two ways of naming ethers.
In the first method (which follows the IUPAC nomenclature of organic chemistry), we start by separating the molecule through the oxygen atom. We list the shorter half of the molecule then insert “-oxy” and then list the longer half ending in “-ane” if all carbons are saturated (meaning they have the maximum amount of hydrogens possible). In this lesson we will only deal with saturated carbons.
In the second method (resulting in the molecule’s common name), we again divide the molecule through the oxygen. Then we list the two halves in alphabetical order, giving the root for the number of carbons in each half and a “-yl” ending. Finally we add the word “ether.”
For example:
| CH3CH2CH2OCH3 | Methoxypropane or methyl propyl ether |
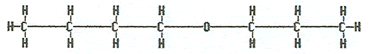 |
Propoxybutane or butyl propyl ether |
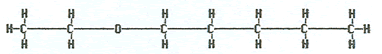 |
Ethoxypentane or ethyl pentyl ether |
Aldehydes contain an oxygen atom doubly bonded to a carbon atom and that same carbon atom needs to have a hydrogen atom attached to it. In practice, that means the double bonded oxygen atom is on the end of the chain.
To name an aldehyde, locate the longest chain containing the double bonded oxygen attached to it, remove the final “e” and replace it with “–al.” With the oxygen always on the end no location number is needed.
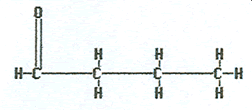 |
Butanal |
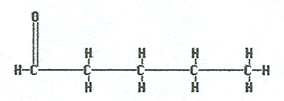 |
Pentanal |
 |
Hexanal |
Ketones contain an oxygen atom doubly bonded to a carbon and that same carbon needs to have carbon atoms attached to both sides of it. In practice, that means the double bonded oxygen atom is not on the end of the chain.
To name a ketone, locate the longest chain containing the double bonded oxygen attached to it, remove the final “e” and replace it with “-one.” If the oxygen can be placed on a carbon atom in the chain where it could have a different location number, then the location must be included. This will be the case when there are five or more carbon atoms in the chain. Note that the shortest chained ketone contains three carbons.
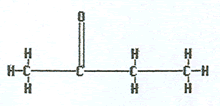 |
butanone |
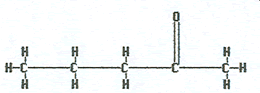 |
2-pentanone |
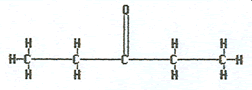 |
3-pentanone |
 |
2-hexanone |
Carboxylic Acids contain an oxygen atom doubly bonded to a carbon and that same carbon needs to have hydroxyl group (-OH) attached to it. In practice, that means the acid functional group is on the end of the chain and therefore does not need a number.
To name a carboxylic acid locate the longest chain that the functional group is attached to and replace the final “e” with “-oic” and acid.
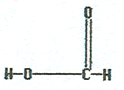 |
Methanoic acid |
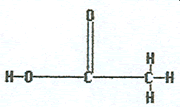 |
Ethanoic acid |
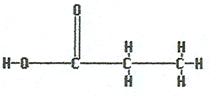 |
Propanoic acid |
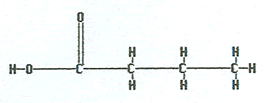 |
Butanoic acid |
What is name of the following molecule?
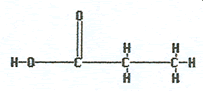
The correct answer is A. This is a carboxylic acid with three carbon in the chain. The “propan-“ is because of three carbons singly bonded together and the “-oic” acid to indicate it is an acid.
What is name of the following molecule?
The correct answer is C. This is a ketone containing seven singly bonded carbon atoms therefore the “heptan-” and the “-one” to indicate it is a ketone. The “2-“ shows that the double bonded oxygen is attached to the second carbon over. The 2 is used since it is smaller than 6 which would be the number if the chain were numbered in the other direction.
What is name of the following molecule?
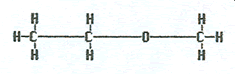
The correct answer is D. This is an ether. When naming an ether, we divide the chain through the oxygen, list the short half of the chain, the “-oxy-“ (for the oxygen), then the longer half of the chain. Or we list the two halves in alphabetical order, with a “-yl” ending and add that it is an ether.
Esters contain an oxygen atom doubly bonded to a carbon, and that same carbon needs to have a singly bonded oxygen attached to it. Finally, that singly bonded oxygen needs to have a carbon attached to its other side. In other words, the H of the –OH group in a carboxylic acid functional group has been replaced by one (or more) carbon atoms.
To name an ester, first name the carbon chain connected to the single bonded oxygen that does not contain the double bonded oxygen and change the ending from –ane to –yl. Next, use the root that describes the number of carbons in the chain attached to the double bonded oxygen adding the ending –anoate.
|
|
Butyl methanoate |
|
|
Methyl butanoate |
|
|
Ethyl propanoate |
|
|
Propyl ethanoate |