 username@email.com
username@email.com
In this lesson we will review how to balance redox reactions. We will also review the Daniell Cell and workings of electrolysis.
Redox (oxidation-reduction) reactions are ones in which electron(s) are transferred. In a redox reaction:
There are several steps we must use to properly balance redox reactions.
Balance the following redox reaction that occurs in a basic solution:
NO2-1( aq) + Al (s) → NH3(g) + AlO21-( aq)
NO2-1(aq) → NH3(g)
NO2-1(aq) → NH3(g) + 2H2O(l)
7H1+(aq) + NO2-1(aq) → NH3(g) + 2H2O(l)
6e1- + 7H1+( aq) + NO2-1(aq) → NH3(g) + 2H2O(l)
Al(s) → AlO21-(aq)
2H2O(l) + Al(s) → AlO21-(aq)
2H2O(l) + Al(s) → AlO21-(aq) + 4H1+( aq)
2H2O(l) + Al(s) → AlO21-(aq) + 4H1+( aq) + 3e1-
This equation will need to be multiplied by two to ensure the electrons will be equal in both reactions and will cancel.
Then add the two half reactions.
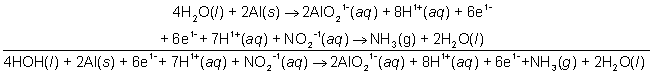
2H2O(l) + 2Al(s) + NO2-1(aq) → 2AlO21-(aq) + H1+(aq) + NH3(g)
If the equation were in acidic solution, this would be the final point, but since in basic solution, we need to add enough hydroxide ions to both sides of the reaction to effectively cancel the hydrogen ions present and create an equal number of moles of water.
OH1-(aq) + 2H2O(l) + 2Al(s) + NO2-1(aq) → 2AlO21-(aq) + H1+(aq) + NH3(g) + OH1-(aq)
Combine hydroxide and hydrogen to form water.
OH1-(aq) + 2H2O(l) + 2Al(s) + NO2-1(aq) → 2AlO21-(aq) + H2O(l) + NH3(g)
Cancel one water from each side.
OH1-(aq) + H2O(l) + 2Al(s) + NO2-1(aq) → 2AlO21-(aq) + NH3(g)
Then check to ensure charges are equal.
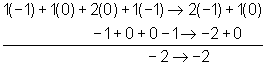
Balance the following reaction in acidic solution using the half-reaction method, and determine how many moles of electrons are transferred.
Br(aq)1- + MnO4(aq)1- → Br2(l) + Mn(aq)2+
The correct answer is D.
Br(aq)1- + MnO4(aq)1- → Br2(l) + Mn(aq)2+
Br(aq)1- → Br2(l)
2Br(aq)1- → Br2(l)
2Br(aq)1- → Br2(l) + 2e1-
When balancing the half reaction with bromine, there are two electrons in the equation.
MnO4(aq)1- → Mn(aq)2+
MnO4(aq)1- → Mn(aq)2+ + 4H2O(l)
8H(aq)1+ + MnO4(aq)1- → Mn(aq)2+ + 4H2O(l)
5e1- + 8H(aq)1+ + MnO4(aq)1- → Mn(aq)2+ + 4H2O(l)
The half reaction with the manganese has five electrons.
The lowest common multiple of two and five is ten.
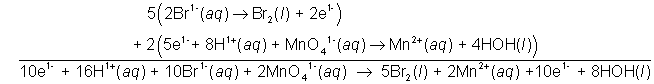
16H(aq)1+ + 10Br(aq)1- +
2MnO4(aq)1- → 5Br2(l) + 2
Mn(aq)2+ + 8H2O(l)
10 mol electrons are transferred.
Balance the following reaction that occurs in acidic solution. Determine the moles of electrons
transferred.
H3AsO4(aq) + Zn(s) → AsH3(g) + Zn(aq)2+
The correct answer is D.
H3AsO4(aq) + Zn(s) → AsH3(g) + Zn(aq)2+
Zn(s) → Zn(aq)2+
Zn(s) → Zn(aq)2+ + 2e1-
H3AsO4(aq) → AsH3(g)
H3AsO4(aq) → AsH3(g) + 4H2O(l)
8H(aq)1+ + H3AsO4(aq) → AsH3(g) + 4H2O(l)
8e1- + 8H(aq)1+ + H3AsO4(aq) → AsH3(g) + 4H2O(l)
One half-reaction shows 2 moles of electrons and the other eight. The least common multiple of two and eight is eight.

Eight moles of electrons are transferred. Now, cancel as needed.
4Zn(s) + 8H(aq)1+ + H3AsO4(aq) → AsH3(g) + 4H2O(l) + 4Zn(aq)2+
The Daniell Cell is a device that uses the energy of a spontaneous redox reaction to produce electric current. It was devised in 1836 by British chemist and meteorologist John Frederick Daniell. The cell used a zinc anode, copper cathode, and solutions of zinc sulfate and copper sulfate. It is the predecessor of the current day galvanic cell. The galvanic cell uses a porous disk or a salt bridge to allow for the flow of ions between half-cells to maintain electrical neutrality.
The Daniell Cell allows for the flow of electrons from the anode to the cathode through an external circuit. Useful work can be done by these electrons flowing through a wire as an electrical current. The anode is the electrode where oxidation occurs and the cathode is the electrode where reduction occurs.
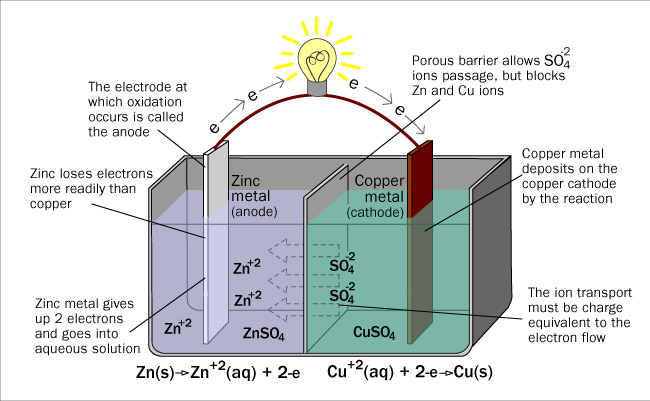
Zn(s) | Zn(aq)2+ || Cu(aq)2+ | Cu(s)
The double line (||) represents the salt bridge or porous disk in the drawing. By convention, the anode half-cell is written on the left, and cathode half-cell on the right. The convention shows the anode material, the anode solution, the salt bridge, the cathode solution and then the cathode material. You can tell from the line notation that the Zn is oxidized and the copper (II) ion is reduced from their respective positions in the notation.
The reaction, Zn(s) + Cu(aq)2+→ Cu(s) + Zn(aq)2+ , can be split into half reactions:
Zn(s) → Zn(aq)2+ + 2e–
Zinc is neutral and loses two electrons, which means it is oxidized.
Cu(aq)2+ + 2e– → Cu(s)
Copper has a charge of positive two, then gains two electrons to form neutral copper, hence it is reduced.
Electrolysis is the process of using electric current to decompose compounds into the separate elements and molecules and has many practical applications.
Electrolysis is used to decompose solid table salt (sodium chloride) in a Down’s Cell. In the Down’s cell, the salt is heated until it is melted and the sodium and chlorine are separated, without being able to react with each other.
Salt water may also be separated using electrolysis. Pure water is easier to reduce than sodium because its reduction potential (which will be explained in next lesson) is much less than that of sodium. The electrolysis of a sodium chloride solution produces hydrogen gas and sodium hydroxide rather than pure sodium and pure chlorine. The sodium hydroxide can then be purified using a mercury cell that allows the solid sodium from the salt to react with water to form hydrogen gas. The mercury cell process, also called the chlor-alkali process, has been replaced recently due to the health hazards associated with mercury. Now, an impermeable membrane is used in place of the mercury that allows only cations to flow through, and the result is pure sodium hydroxide.
In addition, electrolysis is used to purify metals such as copper. Huge slabs of copper are placed in a tank containing aqueous copper sulfate and very thin sheets of pure copper are added to function as the cathode. The copper ions in the solution will form on the pure sheets of copper and the impurities will fall to the bottom of the solution producing 99.95% pure copper.
Electrolysis may also be used to electroplate objects such as tin cans and chrome bumpers. The object to be plated is placed in a solution and serves as the cathode. The anode will be the metal which is to be deposited on the object being plated. An important economic advancement in history due to electrolysis has been the production of aluminum. Aluminum oxide (the main component of the ore bauxite) was too expensive to use since it has a very high melting point. Hall and Heroult, independently, but at about the same time, found that by using the mixture of aluminum oxide and sodium aluminum fluoride, a lower melting point was obtained. This causes the liquid aluminum to be deposited at the cathode as a precipitate. The carbon anode is oxidized and bubbles away as carbon dioxide. The overall chemical reaction is:
2Al2O3 + 3C → 4Al + 3CO2
The process produces 99.5% pure aluminum and uses about 5% of the entire electricity output of the United States. Several aluminum producing plants have recently opened in Iceland, using the natural geothermal power to operate the plants at a reduced cost. Due to the work of Hall and Heroult, the price per pound of aluminum has dropped from $100,000 in the 1850’s to about $1 today.