 username@email.com
username@email.com
In this lesson we will review the arrangement of atoms in molecules, ionic crystals, polymers, and metals.
The positions that atoms occupy relative to each other in a substance—element or compound—depend primarily on the type of bonding found in that substance. We will concern ourselves mostly with covalent bonding and ionic bonding, although we will give some consideration to polymeric substances and metals.
The nature of covalent bonding (polar or otherwise) favors the formation of molecules. Molecules are discrete structures; that is, each has a definite beginning and end. Consider carbon tetrachloride, CCl4. Carbon begins with four valence electrons and each chlorine begins with seven. By sharing some of these electrons with each other, each atom is able to obtain eight valence electrons and satisfy the Octet Rule. The accompanying figure shows a Lewis structure of CCl4 (Lewis structures will be reviewed in greater detail in later lessons). Note that each of the five atoms has eight valence electrons around itself.
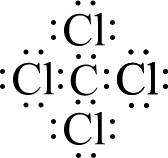
Because the formation of covalent bonds has allowed each atom to attain eight valence electrons, there is no reason for any of these atoms to bond further with other atoms that may be nearby. If more carbon and chlorine atoms are available, they may combine with each other to form more discrete molecules of CCl4, but they will not “add on” to the structure that has already formed. Covalent bonds form strong attachments; therefore, the chlorine atoms are fixed quite firmly to the carbon atom, and will remain bonded to it until chemically removed. The CCl4 molecules, however, are not covalently bonded to each other and thus it is much easier for them to move apart from each other.
When atoms transfer electrons and form ions that attract electrostatically, a very different structure is formed. It is important to remember that when a negative ion is attracted to a positive ion, they will stick together but they retain their charges. For this reason, if other ions are nearby, they will also be attracted to these ions. Each negative ion will surround itself with as many positive ions as physically possible in three-dimensional space (usually four or six ions). Each of these positive ions will then surround itself with as many negative ions as possible. This cycle continues as long as ions are available.
When this pattern is exhibited in three dimensions, the structure formed is an ionic crystal. A simple example is represented in the figure below.
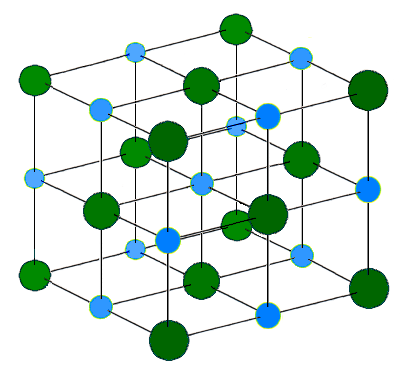
What is really important to note about this structure is that all of the ions in it are somehow interconnected with each other, and that the structure can continue in all directions as long as there are additional ions available to add to it. It is not discrete, but indefinite.
A small handful of substances exhibit a combination of these patterns. They bond covalently, but in a three-dimensional indefinite array rather than in discrete molecules. These substances are referred to as polymers or network covalent substances. Very few substances bond in this manner, but the most customary examples are quite common, indeed. Diamond, graphite (allotropes of carbon), quartz and other oxides of silicon are the most frequently encountered examples of this group.
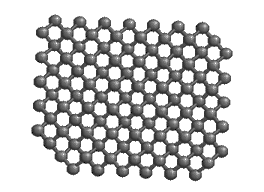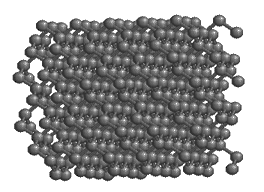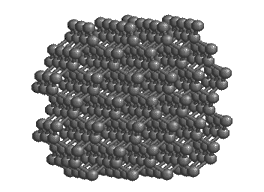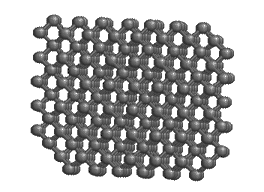
Metals are another example of substances that form a three-dimensional indefinite array of atoms in space. Metals have low ionization energies and low electronegativities, therefore they do not hold onto their few valence electrons particularly well. These electrons tend to “float” from metal atom to metal atom, forming what is sometimes referred to as a “sea of electrons,” freely flowing throughout the metal sample. The attraction of the metal atoms for these wandering electrons holds the metal together.
Which of the following substances forms discrete molecules?
The correct answer is A. Discrete molecules are formed by covalent bonds, which occur between nonmetals. This rules out choices C and D. Choice B also bonds covalently, but it is one of the polymeric/network covalent substances.
Which of the following substances forms a three-dimensional array of oppositely-charged particles?
The correct answer is C. Choices B and D also form 3D arrays, but of neutral atoms. Choice A forms discrete molecules. Choice C has a metal bonding with a nonmetal, so it will form negative and positive ions that will alternate in three-dimensional space.
Which of the following substances forms a three-dimensional array of neutral particles?
The correct answer is C. Choices B and D also form 3D arrays, but of neutral atoms. Choice A forms discrete molecules. Choice C has a metal bonding with a nonmetal, so it will form negative and positive ions that will alternate in three-dimensional space.