 username@email.com
username@email.com
In this lesson we will review percent composition, empirical formulas, and molecular formulas.
Chemical formulas are extremely useful, in that they show us the ratio of atoms in a compound. And because a mole is simply a whole number multiple of atoms, a formula also shows us the ratio of moles. Thus, water’s formula, H2O, lets us know that the compound contains two atoms of hydrogen for every atom of oxygen or two moles of hydrogen for every mole of oxygen.
But in the chemistry laboratory, we often measure mass. And because atoms of different elements can have widely different masses, the ratio of atoms or moles is often radically different from the ratio of masses. For this reason, we sometimes use percent composition.
Percent composition is nothing more than the percent by mass of each element in a compound. When a compound’s formula is known, it is most easily calculated by determining the compound’s molar mass, element by element. We then divide the mass of each element by the total mass of the compound and multiply by 100%.
Consider calcium sulfate, CaSO4. A mole of this compound contains 40.1 g Ca (1 mole), 32.1 g S (1 mole) and 64.0 g O (4 moles), for a total mass of 136.2 g. The percent composition of CaSO4 would be calculated as follows:

When we analyze an unknown substance in the laboratory, we usually obtain mass data first. From that data, we would like to determine the compound’s chemical formula. The first step in this process is to determine the empirical formula.
To find an empirical formula, it is helpful to remember two things. First, the ratio of atoms is the same as the ratio of moles; therefore, any data that we have must be converted to moles. Second, since compounds have definite composition, we may choose any sample size we wish when calculating.
Consider a compound whose composition is 74.5% Pb and 25.5% Cl. Again, any sample size will work, but if we choose 100 grams, our calculation will start off more easily, because the sample will contain 74.5 g Pb and 25.5 g Cl. Our first step is to convert these masses into moles using molar mass, as shown below:

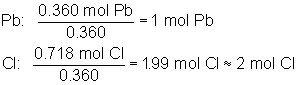
Which of the following is the correct empirical formula for a compound that is 30.4% N and 69.6% O by mass?
The correct answer is B. We may eliminate choice D immediately as its ratio can be reduced, so it is not an empirical formula. The calculation below shows how the empirical formula of NO2 is found:
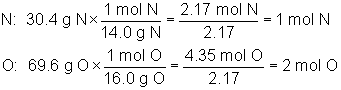
The empirical formula is a valuable first step, but does not provide enough information to be of great use. After all, glucose (C6H12O6), acetic acid (CH3COOH) and formaldehyde (CH2O) all have the same 1:2:1 ratio of C:H:O, but they have vastly different properties. What we really want is the molecular formula.
Let’s consider a compound that is 43.7% P and 56.3% O. We would begin by calculating its empirical formula as follows:
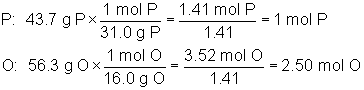
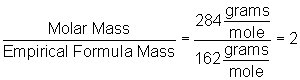
Which of the following is the correct molecular formula for a compound that is 26.7% C, 2.24% H and 71.1% O, and has a molar mass of 90.0 grams per mole?
The correct answer is A. We must first calculate the empirical formula of CHO2 as follows:
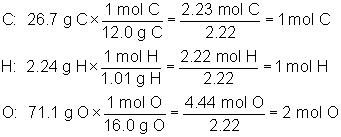
Now we find the whole number multiple by dividing the given molar mass by the mass of the empirical formula that we just calculated, as shown below:

Multiplying the empirical formula through by two gives us C2H2O4.
Which of the following is the correct empirical formula for a compound that is 26.6% K, 35.4% Cr, and 38.1% O?
Choice C is correct. The formula of K2Cr2O7 is found by multiplying by two the numbers from the calculation shown below:

What is the correct molecular formula for a compound that is 57.1% C, 4.81% H, and 38.1% O, and that has a molar mass of 126 grams per mole?
The answer is C6H6O3. The empirical formula is calculated as follows:
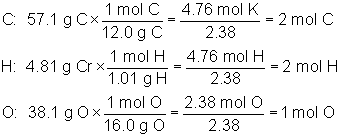
Our empirical formula is C2H2O. Taking the given molar mass and dividing by the mass of the empirical formula will give us the multiple, as follows:
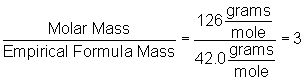
Multiplying the empirical formula by three gives the result, C6H6O3.