 username@email.com
username@email.com
In this lesson we will examine the relationships between acids, bases, and salts and equilibrium in both experimental
situations and common calculations.
When working with weak acids, weak bases, or buffer systems we must take into account the fact that many species exist in the reaction vessel. Take for example the case of calculating the pH of a 0.100 M acetic acid solution whose Ka (acid dissociation constant) is 1.8×10-5.
Since acetic acid is a weak acid, only part of it dissociates so this becomes an equilibrium problem instead of a simple stoichiometry problem (like it would if it were a strong acid).
The reaction is:
HC2H3O2 (aq) + H2O (l) ↔ H3O1+(aq) + C2H3O 21–(aq)
We begin by writing the equilibrium expression:
| HC2H3O2 | H3O1+ | C2H3O21- | |
|---|---|---|---|
| Initial | 0.100 M | 0 | 0 |
| Change < | –x | +x | +x |
| Equilibrium | 0.100 M – x | x | x |
Substituting into the equilibrium expression we get:
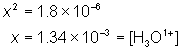
Let’s look at another example.
How would we calculate the pH of a 2.00 M ammonia solution with a Ka= 5.71×10-10 at 25°C?
The reaction is NH3aq) + H2O(l) ↔ NH41+(aq) + OH1–(aq)
Using the equilibrium expression:
Kw = KaKb = 1.0×10–14
Kb (base dissociation constant) = ![]() .
.
Substituting in values we get: Kb = ![]()
| NH3 | NH41+ | OH1- | |
|---|---|---|---|
| Initial | 2.00 M | 0 | 0 |
| Change | –x | +x | +x |
| Equilibrium | 2.00 – x | x | x |
Substituting into the equilibrium expression we get:

pH + pOH = 14
pH = 14 – 2.22
pH = 11.78
A titration is the experimental procedure used to determine the concentration of an unknown acid or base. The necessary chemical components for a titration include a standard solution, a solution of unknown concentration, and an indicator.
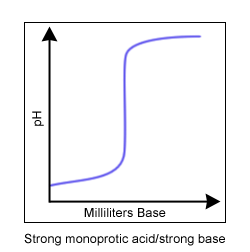
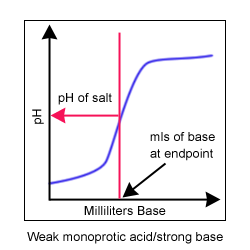
A titration curve is a way to visually represent the change in pH during a titration. Titrations and titration curves tell you many things about a reaction. During a titration, the indicator changes color at the endpoint of the titration. If the best indicator is chosen, the endpoint and the equivalence point (when there are equal amounts of acid and base) occur at nearly the same time.
Common indicators used in acid/base titrations include phenolphthalein (clear to pink pH = 8-10) and bromothymol blue (yellow to blue pH = 6-8). The equivalence point can be visually determined from the titration curve. It is the area where the slope of the line approaches infinity (or is nearly vertical).
 |
 |
 |
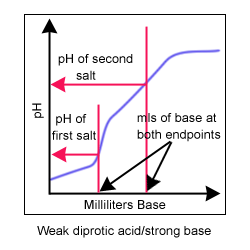 |
Four titration curves. A monoprotic acid is an acid with only one hydrogen ion to donate. A diprotic acid has two hydrogen ions to donate.
Combinations of weak acids or weak bases with a salt containing a corresponding ion form buffers. Buffers are solutions which resist changes in pH when hydrogen or hydroxide ions are added. The amount of acid or base a buffer can neutralize before the pH of the system changes to any appreciable degree is the system’s buffer capacity. The larger the concentration of the buffer components, the larger the buffer capacity.
The Henderson-Hasselbalch equation can be used to solve for the pH of a buffer system.
![]() or
or ![]()
pKa and pKb are −log( Ka) and −log( Kb) respectively
What is the pH of a solution that contains 3.00 moles of ammonia and 2.50 moles of ammonium chloride in 1.00 liter of solution? The Kb of ammonia is 1.81 × 10-5.
The correct answer is D. Using ![]() , we substitute in the given information:
, we substitute in the given information:

Solve for pH using pH + pOH = 14
pH = 14 − pOH
pH = 14 −4.66314 = 9.34