Gas Laws and Solutions: Review
Review
- The KMT states:
- A gas consists of objects with a defined mass and zero volume.
- The gas particles travel randomly in straight-line motion where their movement can be described by the fundamental laws of mechanics.
- All collisions involving gas particles are elastic. No energy is lost and no heat is produced.
- The gas particles do not interact with each other or with the walls of their container.
- Temperature is directly proportional to the kinetic energy of the gas phase system.
- The ideal gas model states:
- Ideal gas particles are so small that the volume of the individual particles if they were at rest would be essentially zero when compared with the total volume of the gas.
- Ideal gas particles are in constant, rapid, random motion, moving in straight lines in all directions until they collide with other particles or the sides of their container.
- There are no attractive or repulsive forces between particles, and collisions between them are elastic.
- The average kinetic energy of the particles is directly proportional to the absolute temperature (measured in Kelvin).
- Real gases are defined as gases that do not fit the kinetic molecular theory.
- The four variables used to describe gases are temperature, pressure, moles, and volume. The gas laws of Graham, Dalton, Charles and Boyle, the combined gas law, and the ideal gas laws are based on the relationships between gas variables.
- Standard temperature and pressure (STP) describes the accepted values for all gas law calculations.
- In order for a solution to form, solute and solvent particles must interact.
- Specific quantitative terms used to describe solution concentration include ppm,
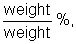
 , molarity and molality.
, molarity and molality.
- Molar concentrations of solutions made through a dilution process can also be calculated using M1V1=M2V2.
- Different types of interparticle interactions occur in solution including ion-ion, ion-dipole, dipole-dipole, hydrogen bonds, and London dispersion forces.
- Diffusion and osmosis are processes in which particles move through various media.
- Concentrations, amounts of solutions, and Ksp values can be used to predict if precipitates will form when solutions are mixed.
- The theoretical freezing and boiling points of a solution can be calculated if you have the molality of the solution and the freezing/boiling point constant for the solvent.
- Raoult’s Law can be used to calculate the vapor pressure over a solution containing a nonvolatile solute over a solution containing two volatile liquids.
- Henry’s Law can be used to predict the concentration of gases in a mixture.
- The Arrhenius Concept defines acids as substances that produce H+ (hydrogen ions) in solution and bases as substances that produce OH– (hydroxide ions) in solution
- The Brønsted-Lowry Model defines acids as H+ (hydrogen ions) donors and bases as H+ (hydrogen ions) acceptors.
- In Lewis’ Theory, acids are electron pair acceptors in solution and bases are electron pair donors in solution.
- pH can be found using either
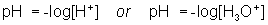
- Water can act as an acid or a base. If you are given either the [H3O1+ ] or [OH1– ], you can solve for the other.
- A weak acid or base is one where only a small portion of the particles dissociate or one that only partially ionizes. A strong acid or base is one that completely dissociates in water, or has 100% ionization.
- When calculating the pH of a weak acid, only part of it dissociates so it becomes an equilibrium problem. To find the pH, write the equilibrium expression,
 , look at the conditions during the reaction, substitute into the equilibrium expression and solve. Use the equation for pH (pH = -log[H3O1+]) and find the pH.
, look at the conditions during the reaction, substitute into the equilibrium expression and solve. Use the equation for pH (pH = -log[H3O1+]) and find the pH.
- A titration is the experimental procedure used to determine the concentration of an unknown acid or base. A titration curve is a way to visually represent the change in pH during a titration.
- Buffers are solutions which resist changes in pH when hydrogen or hydroxide ions are added.
- The Henderson-Hasselbalch equation can be used to solve for the pH of a buffer system.
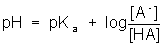 , or
, or 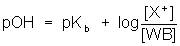
Back to Top
 username@email.com
username@email.com