 username@email.com
username@email.com
In this lesson we will examine how particles interact to form solutions and review the different ways in which concentration is expressed.
Whether a substance is a solution or not depends on the characteristics of the particles involved in the substance. It is important to be able to differentiate between a suspension, a colloid, and a solution. Simple tests can be conducted to determine which substance it is. If particles are visible and settle over time or can be filtered out, it is a suspension. A sample of water taken from the edge of a lake that has been recently disturbed is a good example of a suspension. If the particles are not visible or the mixture seems cloudy and does not settle over time, it may be a colloid or a solution. To determine which it is, shine a light through the material. If the beam of light is invisible, it is a true solution. If you see the light beam reflecting off small particles, you are observing the Tyndall Effect and the substance is a colloid.
Aqueous solutions form when the forces between solute-solvent particles are greater than the forces between solute-solute particles and solvent-solvent particles. In the case of sodium chloride dissolving in water, the forces that hold sodium ions and chloride ions together are ionic bonds. The forces that hold the solvent-solvent particles “together” are the hydrogen bonds between water molecules. When sodium chloride is placed in water, the negative portion of the polar water molecule is attracted to the positive sodium ion. When enough water molecules are attracted to the sodium, they remove the sodium from the crystal lattice. Likewise, the positive portion of the polar water molecule is attracted to the chloride ion and when there are enough water molecules surrounding the chloride ion, it is removed from the crystal lattice.
Solutions can be described with very general terms such as saturated, unsaturated, and supersaturated. Often these terms relate to the amount of solute found in a specific amount of solvent on a solubility curve.
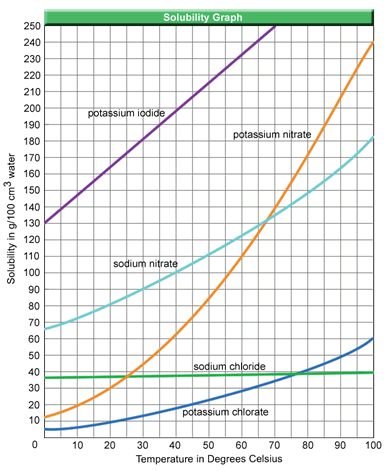
At a given temperature, when the amount of solute dissolved in the given amount of solvent falls below the line for the substance, the solution is unsaturated. When the amount of solute dissolved is greater than the value shown by the line, the solution is supersaturated. If the amount of solute dissolved is equal to the value shown by the line, the solution is saturated.
Based on the graph above, a solution containing 45.0 g of KNO3 in 200.0 cm3 of water at 30ºC would be___________________.
The correct answer is C. The graph shows that at 30 ºC, 45.0 g of KNO3 would be the maximum amount of solute that could be dissolved in 100.0 cm3 of water. Based on this information, if you had 200.0 cm3 of water, 90.0 g of KNO3 could be dissolved, which would be twice the amount given in the question.
Concentration is the term used to describe the amount of solute present in a solution. Concentration can be expressed in a variety of units. When describing very small amounts of a solute in a large amount of solvent, parts per million, or ppm may be used.
Volume/volume% is a unit used when both the solute and the solution can be measured in milliliters. In order to get a 60% alcohol in water solution you would measure out 60 mL of alcohol and dilute it with water until your total volume was 100 mL.
What is the volume/volume concentration of a solution that contains 65.0 mL of rubbing alcohol in 255 mL of aqueous solution?
The correct answer is D. By using the formula ![]() and substituting the values in properly we get:
and substituting the values in properly we get:
![]()
The answer is 25.5 %. If you chose answer C, it is most likely because you added the two volumes (65 mL and 255 mL) to get a larger solution volume. If you selected choice B, you just forgot to multiply by 100 to get the percent. If you picked choice A, you probably used the larger volume and forgot to multiply by 100 to get the percent.
Weight/weight% is a unit used when the weight of the solute can be divided by the weight of the solution and multiplied by 100 to calculate the concentration of the solution.
How much sodium chloride would be necessary to prepare 491 g of a solution whose ![]() concentration is 37.1 %?
concentration is 37.1 %?
The correct answer is C. By using the formula ![]() and substituting the values in properly we get:
and substituting the values in properly we get:
![]()
Solving for the g of solute, the result is 182 g.
The most commonly used unit of concentration in chemistry is molarity, M, which utilizes the number of moles of solute relative to the volume of the solution.
How many grams of sodium sulfate are needed to prepare 500 mL of 0.100 M solution?
The correct answer is D. First, convert the volume from mL to L by dividing by 1000. Next, solve for the number of moles of solute needed by substituting into the equation ![]() to get
to get ![]()
The number of moles necessary will equal 0.0500 moles. To get the number of grams of sodium sulfate, multiply by the molar mass of sodium sulfate. Remember the formula for sodium sulfate is Na2SO4, so the molar mass is 142.00 g/mole (2∙22.99 + 32.06 + 4∙15.99).
Based on molar concentration, or molarity, a simple formula can be employed to determine the concentration of a solution that has been diluted from one of a stronger concentration, sometimes referred to as a stock solution.
M1V1=M2V2
M1 is the concentration of the stock solution and V1 is the volume that you measure to be diluted. M2 is the concentration of the dilute solution and V2 is the volume of your final solution.
Another concentration unit used is molality, m, which compares the number of moles of solute to the mass of the solvent. We will revisit this unit of concentration later when we discuss colligative properties.
How many mL of concentrated hydrochloric acid (HCl) would be necessary to prepare 250.0 mL of 3.00 M HCl?
Concentrated HCl is 12.0 M.
The correct answer is A. Sometimes it is helpful to make a table of the given information to solve a problem.
| Initial | Final |
| M1 = 12.0 M | M2 = 3.00 M |
| V1 = ? | V2= 250.0 mL |
Using the formula M1V1=M2V2, you can solve for the first volume of acid by dividing both sides by M1.