In this lesson we will examine how different types of particles interact in solutions, differentiate between diffusion and osmosis, and see how to predict if precipitation will occur when solutions are mixed.
What Happens When Substances are Added to a Solution?
Interparticle interactions, sometimes called intermolecular forces, explain how substances interact with each other. There are several different types of interparticle interactions with which we should be familiar, including: ion-ion, ion-dipole, dipole-dipole, hydrogen bonds, and London dispersion forces.
-
- Ion-ion interactions are the strongest form of interparticle interactions. These occur when oppositely charged gaseous ions in a vacuum are attracted to each other. As more collisions occur, larger clusters of ions organize and can form a denser gas, a liquid, or even a solid.
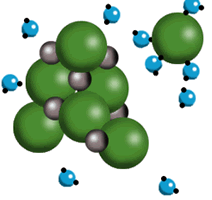
- Ion-dipole interactions are interactions that occur between ions and polar compounds. They are not as strong as ion-ion interactions. These interactions are particularly important to the dissolving of salts in an aqueous solution. The positive sodium ions in sodium chloride are attracted to the partially negative portion of the polar water molecule located around the oxygen. The negative chloride ions are attracted to the partially positive portion of the water located in the regions around the hydrogen atoms.
-
- Dipole-dipole interactions are attractions between molecules with dipoles. Dipole-dipole interactions are weaker than ion-dipole interactions. A molecule like gaseous hydrogen chloride has a pair of dipoles; the highly electronegative chlorine pulls the electron density close to it creating a negative region around the chlorine. When the electron density is pulled away from the hydrogen, it makes the region around the hydrogen partially positive. If two hydrogen chloride molecules approach each other the oppositely charged dipoles are attracted to each other. Dipole-dipole interactions are important in helping prevent some polar solvents from vaporizing easily.
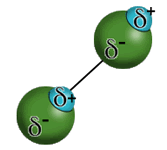
- Hydrogen bonds are a special type of dipole-dipole interactions where a hydrogen atom involved in a polar covalent bond is attracted to a highly electronegative atom, usually fluorine, oxygen, or nitrogen, in another compound. Hydrogen bonding helps to explain why hydrofluoric acid is a weak acid while HCl and HBr are strong acids.
- London dispersion forces are the very weak attractions between nonpolar covalent molecules of the same type such as hydrogen, nitrogen, or diatomic halogens that allow us to liquefy or in the case of iodine, solidify them. The temporary distortion of the electron cloud around an atom causes a temporary dipole, which induces dipoles in other nearby molecules. These interactions inhibit molecular movement as they are attracted to each other and account for the different phases found in the halogens. London dispersion forces are the weakest of the interparticle interactions.
Diffusion and Osmosis
Diffusion is a term often used to describe how one type of particle spreads through another. Here particles move from areas of high concentration to low concentration. When walking through a department store, it is usually easy to notice approaching the fragrance section because the scent diffuses, or spreads, through the air in the store. The particles of low weight diffuse faster than those that are heavier, as expressed in Graham’s Law.
Osmosis on the other hand, describes the process by which solvent particles move through a semipermeable membrane. In osmosis, particles move from areas of low concentration to areas of higher concentration. When a pure solvent and a solution are placed in a container separated by a semipermeable membrane, the solvent particles will travel through the membrane and increase the amount of solution. The process of pickling cucumbers is a good example of osmosis where an area of low concentration (water inside the cucumber) passes through a semipermeable membrane (the “skin” of the cucumber) to a solution of higher concentration (the salt water solution used for pickling). The visible indicator of this process is the shrinkage of the cucumber as it becomes a pickle.
When Solutions are Mixed, Will a Precipitate Form?
When a salt dissolves in water its ionsdissociate. This process can happen very quickly or very slowly. As the ions form, some of them collide and reform the salt until equilibrium is achieved.
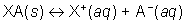 From this equation the solubility product constant, Ksp, can be written as:
From this equation the solubility product constant, Ksp, can be written as:
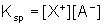 In order to determine if a precipitate will form, the Ksp value must be compared to the ion product or reaction quotient, Q. Q is found in the same way as Ksp, except the initial concentrations of the ions are used instead of the equilibrium concentrations.
In order to determine if a precipitate will form, the Ksp value must be compared to the ion product or reaction quotient, Q. Q is found in the same way as Ksp, except the initial concentrations of the ions are used instead of the equilibrium concentrations.
Conditions:
- When Q > Ksp, then a precipitate will form.
- When Q = Ksp, then the solution is saturated.
- When Q < Ksp, then no precipitate forms.
Sample Problem
Will a precipitate form when 0.30 L of 0.0500 M Na2SO4 and 0.20 L of 0.100 M Pb(NO3)2 are combined? The Ksp of PbSO4 is 6.3 × 10-7.
To solve, find the concentration of both ions in the combined solution. The total volume of the solution is equal to 0.20 L + 0.30 L = 0.50 L
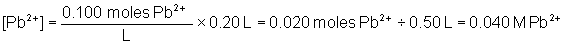
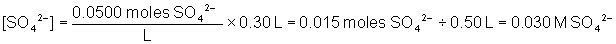
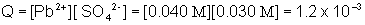
Q is greater than Ksp, therefore a precipitate will form.
Question
Will a precipitate form when 0.010 L of 4.0×10–3 M sodium fluoride is mixed with 0.020 L of 1.0×10–2 M calcium nitrate. The Ksp of calcium fluoride is 3.9×10–11.
- Yes, because Q is greater than Ksp.
- Yes, because Q is less than Ksp.
- No, because Q is greater than Ksp.
- No, because Q is less than Ksp.
Reveal Answer
The correct answer is A.
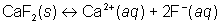
The total volume of the solution is equal to 0.010 L + 0.020 L = 0.030 L
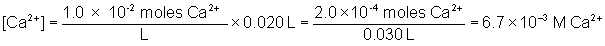

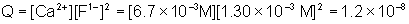
Q is greater than Ksp, therefore a precipitate will form.
 username@email.com
username@email.com


![]()
![]()
![]()
![]()