 username@email.com
username@email.com
In this lesson we will review nuclear structure and stability. We will also review balancing nuclear equations.
All atoms are composed of three subatomic particles, protons, neutrons, and electrons, the masses and charges of which are given in Table 1. The protons and neutrons, called nucleons, are located in the nucleus, at the center of the atom. The electrons occupy the region around the nucleus. A typical atom has a radius of about 1×10−10 m, but the radius of a nucleus is of the order of 1×1015 m. This means that while the protons and neutrons in the nucleus make up more than 99.9 percent of the mass of a typical atom, they occupy only 1 part in 10−15 of its volume!
| Particle |
Symbol |
Charge* |
Mass (kg) |
| Electron |
e– |
1– |
9.10939 ×10–31 |
| Proton |
p |
1+ |
1.672623 × 10–27 |
| Neutron |
n |
0 |
1.674929 × 10–27 |
| *The charge is given relative to the magnitude of the charge on an electron, 1.602×10–19 C (Coulomb, the SI unit for charge). |
|||
Objects with opposite electrical charges attract each other; objects with like charges repel each other. Coulomb’s electrical force law, ![]() , where q1 and q2 are the charges and r the distance between the charges, describes the magnitude of the electric force between two point-like charges, and predicts that the repulsive force between positively charged protons increases as the distance between them decreases. The very tiny dimensions of the nucleus means that the positively charged protons in the nucleus are exceedingly close to one another, and so the electrical force pushing them apart must be extremely large.
, where q1 and q2 are the charges and r the distance between the charges, describes the magnitude of the electric force between two point-like charges, and predicts that the repulsive force between positively charged protons increases as the distance between them decreases. The very tiny dimensions of the nucleus means that the positively charged protons in the nucleus are exceedingly close to one another, and so the electrical force pushing them apart must be extremely large.
An attractive force, the strong nuclear force, is responsible for holding the nucleus together. While this force is sufficiently strong to hold protons and neutrons together in the nucleus, it is a short-range force and has essentially zero magnitude at separations greater than the dimensions of the nucleus. Neutrons, which have no electrical charge, contribute to nuclear binding, and as the number of protons in the nucleus increases, the number of neutrons also increases. For the first 20 elements, the neutron-proton ratio is very close to 1:1; for elements with more than 20 protons, the ratio is greater than 1:1 and increases with increasing atomic number.
Bringing protons and neutrons together to form a nucleus always releases energy—the nucleus has a lower energy than the uncombined protons and neutrons—and the nucleus formed always has less mass than that of the protons and neutrons that make it up. During such a reaction the lost mass, or mass defect, is converted into energy: the nuclear binding energy. The nuclear binding energy can be calculated using Einstein’s mass-energy relationship, ∆E = ∆mc2, where ∆E is the binding energy, ∆m is the mass defect, and c is the speed of light.
If we divide the binding energy by the number of nucleons in the nucleus and plot this ratio vs. the atomic number (see graph below), we find that the most stable nucleus is the one with the largest amount of energy released per nucleon, Fe–56. (Since formation of nuclei from nucleons is exothermic, binding energies are negative.)
Nuclei with atomic numbers smaller than 26 (the atomic number of Fe–56) can undergo fusion to form heavier, more stable nuclei with a release of energy. Nuclei with atomic numbers larger than 26 can undergo fission to form lighter, more stable nuclei, also with a release of energy.
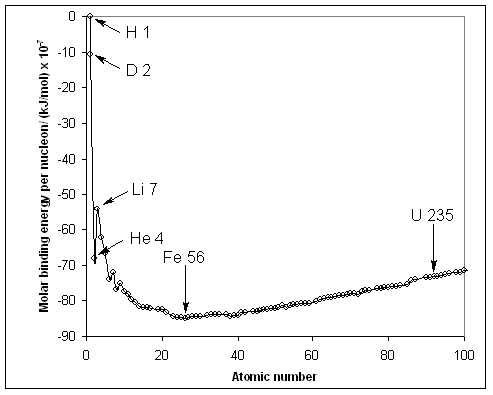
Ratio of binding energy to the number of nucleons in the nucleus vs. the atomic number
For example, when a He–4 nucleus fuses with a C–12 nucleus to form O–16, 69.1×107 kJ/mol of energy is released, and when a U–238 nucleus splits into a Th–234 nucleus and an alpha particle, 83.5×107 kJ/mol of energy is released.
Is fission of Cs–55 into Fe–26 and Cu–29 energetically favorable?
The correct answer is yes. Both Fe–26 and Cu–29 are more stable than Cs–55, so energy would be released.
For nuclei to form from protons and lighter nuclei, reactant particles must collide with sufficient energy to overcome repulsive electrical forces and approach each other closely enough for the strong nuclear force to bind them together. At extremely high temperatures and pressures, collisions become energetic and frequent enough for fusion to occur. Such conditions existed for a few minutes after the Big Bang, during which nuclei as heavy as helium were formed, and they exist in stars and supernovas, in which heavier elements are formed. The very heaviest elements in the Universe, such as actinium, thorium, and uranium are created in explosions of supernovas.
As of 2004 there were nearly 3,000 known nuclides. Most are man-made and undergo radioactive decay. Of the 287 nuclides found in nature, 266 are stable; the remaining 21 are radioactive. There are a variety of radioactive decay pathways by which unstable, radioactive parent nuclei are transformed into one or more daughter nuclei. These decay processes involve emission of subatomic particles and/or radiation. While there are a variety of subatomic particles involved in nuclear reactions, for our purposes we need only consider protons, neutrons, electrons, and positrons. (Positrons are the antimatter counterpart of electrons, with the same mass as electrons but a positive charge.)
Decay of unstable nuclei is a random process—the moment at which any particular nuclei in a sample will decay is unpredictable and the number of radioactive nuclei in a sample diminishes exponentially with time. Each radioactive species has a characteristic half-life — the time period during which the number of radioactive nuclei decreases by half. Since the generally accepted age of the earth is 4.6 billion years, the only naturally occurring radioactive isotopes, or radioisotopes, will be those in one of the following three classes:
Alpha decay, the emission of a helium nucleus (or α particle) is a major decay path for elements with atomic numbers 83 or higher, and a minor decay pathway for lighter nuclei. All nuclei with atomic numbers greater than 83 are unstable with respect to alpha decay; the mass of the parent nucleus is greater than the combined mass of the daughter nucleus and alpha particle. A noteworthy example of alpha decay is the conversion of polonium–210, to lead–206.
![]() →
→ ![]() +
+ ![]()
α decay
Alpha particles can travel only a few centimeters in air and are stopped by a layer of tissue a few cells thick. However, because of the large mass and charge of helium nuclei, alpha radiation causes extensive ionization in living tissue and is the most damaging form of radioactivity, so if alpha sources are ingested, radiation damage can be severe.
Electron emission (or β– decay) in which a neutron inside the nucleus changes into a proton with emission of an electron is the mode of beta decay most commonly discussed in introductory textbooks. There are two other radioactive decay processes classified as beta decay, β+ decay, in which a proton inside a nucleus changes into a neutron with the emission of a positron; and electron capture, in which a proton in the nucleus changes into a neutron by capturing an inner shell atomic electron, usually a 1s electron. For each of these nuclear processes, the mass number A remains constant, while Z changes by ±1.
Beta decay is the principal decay path of radioisotopes, especially those with atomic numbers less than 83. An example of β– decay is the decay of a C–14 nucleus into an N–14 nucleus, the basis of carbon–14 dating.
![]() →
→ ![]() +
+ ![]()
β decay
Beta particles can penetrate through the skin to depths of a few millimeters and damage living cells in their path.
In γ (gamma) decay, a nucleus in an excited or metastable state decays to a lower energy state by emission of a high energy photon, or gamma ray. Gamma ray emission usually follows alpha or beta decay, as a product daughter nucleus decays to a lower energy state by emission of one or more γ rays. An example of a nuclear process involving gamma rays is the decay of Co–60 to Ni–60. The first step in this reaction is the beta decay of Co–60 to a metastable, excited Ni–60 nucleus.
![]() →
→ ![]() +
+ ![]()
This is followed by emission of two gamma rays in succession.
![]() →
→ ![]() + γ1 + γ2
+ γ1 + γ2
γ decay
Gamma radiation can pass completely through the body, leaving a trail of damaged cells in its wake. Focused gamma radiation is used in radiation therapy to destroy cancerous tumors.
There are two modes of radioactive decay: spontaneous fission or natural radioactivity, and induced fission or artificial radioactivity. Radioactive isotopes, such as Th–233, decay into lighter nuclides spontaneously.
![]() →
→ ![]() +
+ ![]()
Induced fission, or artificial radioactivity, was first observed by Irene Curie and her husband, Pierre Joliot, in 1934. When they bombarded aluminum foil with α particles produced by the decay of polonium, they discovered that a radioactive isotope of phosphorus was formed:
![]()
Radioactive P–30 then decays by positron emission:
![]()
Today there are more than 2,000 artificial radionuclides that have been synthesized.
Nuclear reactions produce both particles and energy. In a nuclear reaction, charge, mass number (A), and atomic number (Z) are all conserved.
Reactants and products may include:
There are two conventions used in writing nuclear reactions you should keep in mind. The mass number of electrons and positrons is zero, and the charge on a nucleus is usually not included. Two worked examples of balancing nuclear reactions are given below, the first for a fission reaction, and the second for a fusion reaction.
A Fe–60 nucleus can decay to form two beta particles and a new nucleus: ![]()
What is the identity of this new nucleus ![]() ?
?
Conservation of mass numbers means the sum of the mass numbers of reactants must equal the sum of the mass numbers of the products. Thus, for this reaction the mass number of ![]() is equal to the mass number of
is equal to the mass number of ![]() plus that of 2 electrons, so
plus that of 2 electrons, so
60 = AX+2(0)
AX = 60 – 0 = 60
Conservation of atomic numbers means the sum of the atomic numbers of reactants must equal the sum of the atomic numbers of the products, so
26 = 2(–1) + ZX
ZX = 26 – (– 2) = 28
Thus, X is a nickel nucleus. Our balanced equation is: ![]()
Check the answer. The sum of mass numbers is 60 (60 = 0 + 60), and the sum of atomic numbers is 26 (26 = 28 – 2) for both products and reactants.
In stars like our Sun, protons can combine to form helium nuclei. Balance the following skeleton reaction for this process.
![]()
In this unbalanced equation the sum of reactant mass numbers is 1 and that of products is 4. We need 4 protons to balance mass numbers, so our equation becomes
![]()
At this point the sum of reactant atomic numbers is 4 and that of products is 3, so we need 1 more positron to balance our equation.
![]()
Check the answer. The sum of mass numbers is 4, (4 = 4 + 0), and the sum of atomic numbers is 4, (4·1 = 2 + 2·1) for both products and reactants.
Balance the following nuclear reactions and identify each as either a fusion or a fission reaction.
a) ![]() (fusion) Balancing mass numbers of reactants and products gives us A + 3 = 4 + 2·1 = 6, so A = 3
(fusion) Balancing mass numbers of reactants and products gives us A + 3 = 4 + 2·1 = 6, so A = 3
Balancing atomic numbers of reactants and products gives us Z + 2 = 2 + 2·1 = 4, so Z = 2
b) ![]() (fission) Balancing mass numbers of reactants and products gives us 235 = 139 + A + 2·1, so A = 94
(fission) Balancing mass numbers of reactants and products gives us 235 = 139 + A + 2·1, so A = 94
Balancing atomic numbers of reactants and products gives us 92 = 56 + Z + 2 · 0, so Z = 36
It is a general result of Einstein’s theory of special relativity that the mass of a system of particles held together by attractive forces is less than that of the separated particles. This is true for chemical as well as nuclear reactions, and means that in exothermic reactions of any kind the mass of the products is less that that of the reactants. Conversely, in endothermic reactions the mass of products is greater than that of reactants.
Can we measure these changes in mass? We can compare the predicted mass change for a hypothetical reaction of hydrogen atoms to form helium atoms (in an actual nuclear reaction, reactants are nuclei, not atoms) with the predicted mass change for an actual chemical reaction, hydrogen atoms combine to form hydrogen molecules.
The balanced equation for the formation of helium atoms from hydrogen atoms is 4 ![]() (4 protons + 4 electrons) →
(4 protons + 4 electrons) → ![]() (2 protons + 2 electrons + 2 neutrons)
(2 protons + 2 electrons + 2 neutrons)
In this reaction two of the four protons combine with two of the four electrons to form two neutrons; these combine with the two remaining protons to form the helium nucleus. A helium atom, with two protons, two neutrons, and two electrons, has a smaller mass than the four hydrogen atoms from which it is formed, and a hydrogen molecule has a smaller mass than the two hydrogen atoms from which it is formed. Let’s compare the mass loss when 1 mole of hydrogen atoms is converted to helium by nuclear fusion to that when 1 mole of hydrogen atoms is converted to hydrogen molecules.
When 1 mole of hydrogen atoms is converted to 1 mole of helium, about 6.2×1011 J of energy is released. When 1 mole of hydrogen atoms is converted to hydrogen molecules, about 2.2×105 J of energy is released. We can use Einstein’s mass-energy relationship to calculate the mass changes for each of these reactions.
Mass loss in fusion of 1 mole of hydrogen atoms to form 1 mole of helium:
In the preceding section we used Einstein’s mass-energy relationship to determine mass changes from the amount of energy released in a reaction. Given masses of reactants and products, we can do this calculation in reverse: from the change in mass that results from a reaction, we can determine the amount of energy released. In the next two examples we calculate the amount of energy released when a mole of Fe–60 nuclei decays to form a mole of Ni–60 nuclei and two moles of electrons (β particles), and when four moles of hydrogen nuclei (protons) fuse to form a mole of helium nuclei and two moles of positrons (β+ particles).
The balanced equation for the first reaction, in which two neutrons in the Fe–60 nucleus decay to form two protons and two electrons, is
Is fission of Cs–119 into Fe–56 and Cu–63 energetically favorable? To answer this question, calculate the change in mass that would occur.
One mole of Cs–119 nuclei has a mass of 0.11889232 kg, a mole of Fe–56 nuclei has a mass of 0.05592068 kg, and a mole of Cu–63 nuclei has a mass of 0.06292370 kg. The mass change is –0.00004795 kg. Since mass decreases in this reaction, it is possible.
Is the reaction ![]() possible? The mass of one mole of Fe–60 is 0.05993408 kg, that of one mole of Co–60 is 0.05993382 kg, and that of a mole of electrons is 0.00000055 kg.
possible? The mass of one mole of Fe–60 is 0.05993408 kg, that of one mole of Co–60 is 0.05993382 kg, and that of a mole of electrons is 0.00000055 kg.
The mass change when one mole of Fe–60 reacts to form one mole of Co–60 and one mole of electrons is +0.00000029 kg. Since the mass change is positive, this reaction is not possible.
Since both nuclear fusion and nuclear fission reactions release energy, both have potential as energy sources, although there are both practical and environmental issues with each.
Therefore, it is easy to understand the attractiveness of fusion as an abundant source of relatively safe energy. The problem with fusion is that though large amounts of time and money have been devoted to its development, no one has yet succeeded in achieving a sustained fusion reaction that produces more energy than it uses.
One of the products of nuclear reactions is heat. In a nuclear electrical power generating plant this heat is used in the same way as in oil, coal, or natural gas electrical power generating systems. It generates steam that drives turbines connected to electrical generators. Presently, all nuclear power plants are based on energy derived from a sustained nuclear fission chain reaction, using either U–235 or Pu–239 as fuel.
U–235 and Pu–239 are both what are termed fissile materials. When they absorb a neutron, they split into two smaller
nuclei and release two or more neutrons on average. If conditions are such that one or more of these neutrons, on average, splits another uranium or plutonium nucleus, a sustained chain reaction can be achieved. There are a great
number of possible fission pathways for each isotope. One example of neutron-induced fission of U–235 is:
![]()
In a nuclear bomb, the nuclear chain reaction is uncontrolled and the result is a nuclear explosion; in a nuclear generator, the chain reaction is controlled, and serves as a stable source of power.
More than 99% of naturally occurring uranium is U–238; less than 1% is U–235. This is significant, because U–238 is not a fissile isotope, and cannot be used as a fuel for nuclear reactors. However, when U–238 absorbs a neutron, it decays into Np–239 which then decays to form Pu–239 by a two-step process:
![]()
![]()
Non-fissile U–238 can be converted into fissile Pu–239 in breeder reactors, nuclear reactors that produce more fuel than they use. While the possibility of using breeder reactors to produce vast amounts of plutonium for use as fuel in nuclear power plants has great appeal for some, the fact that plutonium is generally regarded as the most toxic substance known and is a key component of nuclear weapons causes many others considerable concern.