 username@email.com
username@email.com
In this lesson we will discuss a brief history of the development of the periodic table, and how to use the modern periodic table to obtain information about the atoms of the known elements.
By the early-1800s, approximately 60 elements had been discovered, and chemists were overwhelmed with the task of categorizing the properties of so many new elements. There was no method for accurately determining atomic masses, and chemists used different mass numbers in their work, making the results of one chemist’s work difficult, if not impossible, to be interpreted by another. What was needed was a standardized method for determining atomic mass, and a system of classification of the elements grouping them by similarities.
A number of chemists began to observe relationships among the properties of certain elements. One of the earliest attempts to organize the elements based on their chemical and physical properties was made by the German chemist, Johann Dobereiner. He observed that certain elements could be grouped together in threes based on their similarities. One example was calcium, strontium, and barium; another group was chlorine, bromine, and iodine. In each group, the atomic weight of the middle element was the approximate average of the atomic weights of the other two elements. The grouping of elements into triads appeared to Dobereiner to be more than a mere coincidence, and he developed the law of triads from 1817 to 1829.
From 1863 to 1865, English chemist John Newlands found that when the elements were arranged according to increasing atomic mass, there was a repeating pattern relating to the chemical properties of the elements. Beginning with hydrogen, he observed that every eighth element had similar properties. Because an octave is a group of musical notes that repeats every eighth note, Newlands named his discovery the law of octaves. The law of octaves was not readily accepted because it did not work for all of the known elements. In addition, Newlands was criticized for his analogy of elements to musical notes by fellow scientists who thought it to be unscientific.
As the result of the Karlsruhe Conference held in Germany in 1860, the atomic masses used by chemists throughout the world were standardized. Using the standards from the conference, the Russian chemist, Dimitri Mendeleev, created a table of the 60 elements known at the time by arranging them by increasing atomic mass. When organized this way he noticed the periodic pattern in their properties and formulated the periodic law. However, his procedure left several empty spaces in his periodic table. Undaunted, he predicted the existence, as well as the properties, of the unknown elements that would be found to fill these spaces. Mendeleev’s predictions were proven to be correct with the discoveries of scandium, gallium, and germanium.
Mendeleev’s periodic table was not completely accurate, and new discoveries indicated that several of the elements in his table were not in the correct order. In 1913, the English chemist Henry Moseley found that by arranging the elements in the periodic table by atomic number rather than atomic mass, the problems with the order of the elements was resolved.
Mendeleev is generally credited with the production of the first periodic table, but it was hardly a solo effort. Many scientists contributed, and there is even some disagreement concerning who deserves credit for being the “father” of the periodic table. Mendeleev and German chemist Lothar Meyer, working independently of one another, both produced remarkably similar results concerning the periodic nature of the elements. Unfortunately for Meyer, Mendeleev’s table was published first in 1869 and Meyer’s not until 1870.
Other major changes to the periodic table resulted from the work of American chemist Glenn Seaborg in the 1940’s. He and his colleagues discovered plutonium and the transuranium elements through element 102. In addition, he identified over 100 previously unknown isotopes which increased the accuracy of the atomic masses of the elements. Dr. Seaborg received the Nobel Prize for Chemistry in 1951, and was further honored by having element 106 (Seaborgium) named in his honor, the only element named for a living chemist. Seaborg was also responsible for the fact that, in the modern table, the elements lanthanum and actinium are no longer found in the Lanthanide and Actinide series of elements. His work established these elements to be closer in nature to the transition elements than to the inner transition or rare earth elements.
The periodic table has gone through many modifications since the time of Mendeleev. Significant changes include the rearrangement of the elements by atomic number rather than atomic mass, inclusion of the noble gases, and the addition of more than 40 newly discovered or created elements. However, the basic structure of the periodic table has survived, and every known element today can be placed in the periodic table in a group of elements with similar properties.

The horizontal rows of the periodic table are called periods and the vertical columns which contain elements with similar chemical and physical properties are called families (or groups). This arrangement forms regions in the table that contain elements that are classified as: metals, nonmetals, and metalloids or semimetals.
It is important to note here that there are many forms of the periodic table conveying specialized information about each element. Therefore, you may find slightly different periodic tables than the one shown above in textbooks and on the internet.
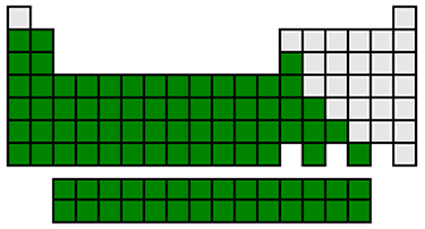
Metals shown in dark green
The majority of the elements are metals. They are generally grouped on the left side of the periodic table. Metals are usually shiny, solid at room temperature, good conductors of heat and electricity, malleable and ductile. The metals can be divided into five subgroups: alkali metals, alkaline earth metals, transition metals, post-transition metals, and inner transition metals.
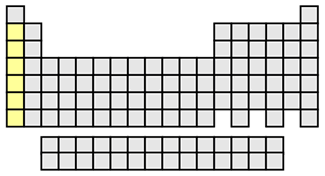
Alkali metals shown in yellow
Group 1 elements except for hydrogen are known as alkali metals. These metals get their name because they react with water to form alkaline or basic solutions. Since the alkali metals are highly reactive, they are never found as elements in nature, but they are always found bonded to other elements. These metals have atoms with only one s electron in their outermost energy level and readily lose that electron in forming ionic chemical bonds with non-metallic elements. The alkali metals form ions with a +1 charge in these bonds. The alkali metals are soft metals, and have low melting points. They react so vigorously with oxygen and water that they are usually stored in oil or kerosene to protect them from the oxygen and moisture in the air.
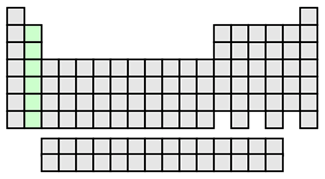
Alkaline earth metals shown in light green
Alkaline earth metals are found in group 2, and also form basic solutions when they react with water. The alkaline earth metals have two electrons in their outermost energy level, completing the s energy sublevel and giving them a little more stability in the metallic state than the alkalis. This group forms ionic bonds by losing their two outermost electrons and forming a cation with a +2 charge when bonding with nonmetals. The alkaline earth metals are harder than the alkali metals. They have a gray-white luster that tarnishes quickly in air as the surface oxidizes. Alloys of these metals can be used as low density structural materials.
Transition metals shown in green
The transition metals are found in the middle of the periodic chart occupying groups 3 through 12 (or on some tables the groups designated with a B). As with all metals, the transition metals are good conductors of heat and electricity, malleable, and ductile. With the exception of liquid mercury (Hg), the transition metals are hard solids with relatively high melting and boiling points. This area of the periodic table is also called the d-block because most of these elements have an incomplete d sublevel.
Post-transition metals shown in blue
There are a few metals located between the transition metals and the nonmetals in groups 13, 14, and 15 arranged in a stair step pattern. The metals are sometimes referred to as post-transition metals. Included in this group are aluminum (Al), gallium (Ga), indium (In), tin (Sn), thallium (Tl), lead (Pb), and bismuth (Bi). These metals tend to have melting and boiling points that are lower than the transition metals and they are generally softer. Their outermost electrons fall into the p-block.
Inner transition metals shown in purple
The inner transition metals (rare earths) contain two groups, the lanthanide series and the actinide series. These elements are usually found along the bottom of the periodic table. The lanthanides are metals with relatively high melting points. They are found mixed together in nature and are difficult to separate because their properties are so similar. The actinides are all radioactive. Only three of these elements are found in nature, and the rest are man-made elements known as transuranium elements. With the exception of plutonium-239, most transuranium elements decay quickly. These elements have a complicated chemistry because they generally contain three incomplete electron energy levels, f, d, and p. On the periodic table they fall into what is also known as the f-block.
Metalloids are shown in gold
The metalloids are found on the periodic table in a stair-step configuration between the metallic elements and the nonmetals. Sometimes called the semimetals, these elements have the physical and chemical properties of both metals and nonmetals and are semiconductors. Silicon and germanium are the two best known metalloids, since they are used in the production of transistors, computer chips, and solar cells.
Nonmetals are shown in orange
Nonmetals are located on the upper right side of the periodic table, except for the nonmetal hydrogen. Other than bromine which is a liquid at room temperature, nonmetals are primarily gases, brittle solids, or crystalline solids. They are generally poor conductors of heat and electricity with varying chemical reactivity. Nonmetals gain electrons when they react with metals and form anions (negative ions) in ionic bonds. Unlike metals, nonmetals will react with other nonmetals by sharing electrons and forming covalent bonds.
Halogens are shown in red
The nonmetals found in group 17 are called halogens. Chemically these are highly active elements which are never found free in nature. The halogens all have 7 electrons in the outermost energy level (p sublevel) and all are extremely aggressive in finding electrons to complete the stable octet which gives each halide ion the configuration of a noble gas. The halogen family elements exist in all three states: F and Cl are gases at room temperature, Br is the only liquid nonmetal, and iodine is a solid.
Noble gases are shown in pink
The elements in group 18 are commonly called the noble gases. The former designation for this group was inert gases because they were thought to be completely inactive chemically. Some of the larger noble gases have been forced into compounds which are very unstable but which prompted the name change. This group has eight electrons in the outermost energy level (completed s and p energy sublevels) which is the point of most stability for the atoms.
A vertical column of elements in the periodic table is referred to as a family or group. The elements in a group have similar chemical properties; and of the three subatomic particles, electrons play the largest role in determining these properties. Therefore, there must be some relationship between the electron configuration of elements and their arrangement in the periodic table. In fact, each family of elements contains the same number of electrons in the outermost energy level. If the electron configurations and the positions of the elements in the periodic table are considered together, the periodic table can be divided into sections or blocks representing the atom’s sublevels being filled with valence electrons. Since there are four different energy sublevels (s, p, d, and f), the periodic table can be divided into four distinct blocks as seen below.
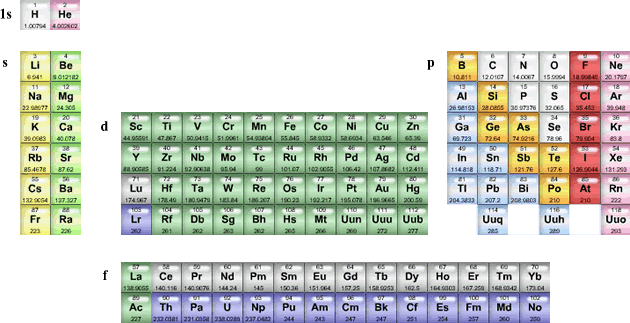
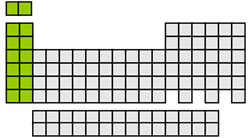
s-block elements
The s-block elements include the alkali metals and the alkaline earth metals plus hydrogen and helium. In this block, the valence electrons occupy only s orbitals. The atoms of group 1 or the alkali metals have only a single valence electron in the s orbital, and the atoms of group 2 or alkaline earth metals elements have a completely filled s orbital containing two valence electrons. The elements of the s-block are all very reactive metals.
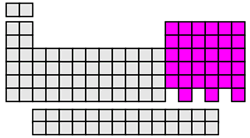
p-block elements
After the s orbital has been filled, the valence electrons next occupy the p sublevel and its three orbitals. The p-block elements include groups 13 through 17. Elements in this block contain atoms with filled or partially filled p orbitals. Since the p-block includes metals, metalloids, and noble gases; the properties of the p-block elements vary greatly.
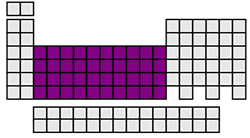
d-block elements
The d-block elements consist of the transition metals. In d-block elements, the filling of the s sublevels in the alkaline earth metals is followed by the addition of electrons to the d sublevels of the previous energy level. These elements have an incomplete principal energy level and incomplete d energy sublevel as well. Many of theses elements can exist in several oxidation states. For example, iron (Fe) can have the oxidation number of 0, +2, +3, and +6.
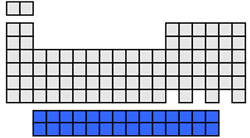
f-block elements
The f-block elements contain the inner transition metals which include the lanthanide series and the actinide series. The f-block elements have filled or partially filled f orbitals. The lanthanides are shiny reactive metals and all of the actinides are unstable, radioactive metals.
Many properties of the elements in the periodic table tend to create a pattern in a predictable way when moving across a period (periodic trend) or up or down a family (group trend).
The area of the electron cloud of an atom is based on probability and does not have a sharply defined boundary to determine its size. However, the atomic size is defined as half the distance between the nuclei of identical elements that are chemically bonded together or half the distance between the nuclei of identical adjacent atoms in a crystal.The increased distance between the outer electrons and the nucleus results in a larger atomic radius.
Unexpectedly, the atomic radii generally decrease from left to right across a period. This trend is caused because the principal energy level within a period remains the same distance from the nucleus but increases from one atom to the next by one more negative charge. The positive charge in the nucleus also increases from one atom to the next due to the increasing number of protons. Moving across a period, no additional electrons come between the nucleus and the outermost electrons. Therefore, the outermost electrons are not shielded from the attractive force of the nucleus. The result is that the increasing electrostatic charge on the electrons in the same energy level tends to pull them closer to the nucleus; and thus, the atomic radii decreases.
As in the period trend, the positive charge in the nucleus increases in the atoms as the atomic number increases. However, the electrons are added to successively higher energy levels. The positive charge in the nucleus is increasing, but so too is the distance of the outermost electrons from the nucleus. Therefore, the increased distance offsets the increased pull of the nucleus. Also, in moving down the group, the number of inner electrons increases, and they shield the outermost electrons from the pull of the nucleus. As a result, the atomic radius generally increases from the top to the bottom of a group.
Atoms form ions by either gaining or losing one or more electrons. If an atom loses an electron, it becomes a positively charged ion and becomes smaller in size. The decrease in size is due to the fact that the lost electron will be a valence electron and may leave the outer orbital completely empty resulting in a smaller radius. The radius of the atom will also be reduced even if the loss of an electron does not completely empty the outer orbital. With the loss of a valence electron, the repulsion of the remaining outermost electrons will be reduced, allowing them to be pulled closer to the nucleus. When atoms gain electrons, they become negative ions and increase in size. The addition of an electron increases the electromagnetic repulsion of the atom’s outermost electrons which forces them to move farther apart. The increased distance between the electrons results in a larger radius.
Within each period, the metals on the left form smaller positive ions, and the nonmetals on the right form larger negative ions.
In moving down the group from top to bottom, an ion’s outer electrons are in higher principal energy levels, resulting in an increase in ionic size. This trend is true for both positive and negative ions alike.
Ionization energy is the energy required to remove one electron from an atom of an element. In this way, chemists are able to compare how easily atoms of different elements can give up electrons. Nonmetals have high ionization energy values and will not easily give up electrons, so will be unlikely to form positive ions. Conversely, metals have low ionization energy values and will more readily lose electrons and form positive ions.
Ionization energy values generally increase from left to right across a period. This increase is caused by the increasing nuclear charge, which more strongly holds on to the outermost electrons.
Ionization energy values generally decrease from top to bottom in the groups. This decrease is caused by the increasing atomic radii. Because the outermost electrons are farther from the nucleus with more electron shielding, less energy is required to remove them.
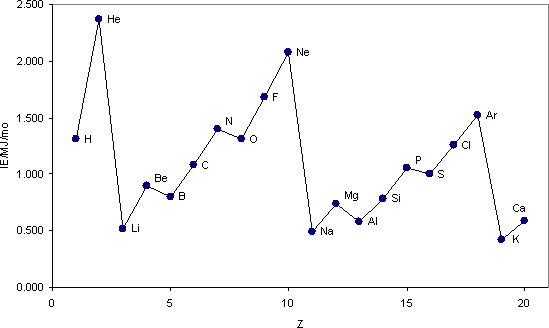
The first ionization energy for the first 20 elements varies by atomic number (Z)
Electronegativity is a relative measure of the ability of an atom to attract electrons when forming a chemical bond. The most electronegative element, fluorine, is arbitrarily assigned a value of four. The other elements are assigned electronegativity numbers relative to fluorine. In a chemical bond, an atom with the largest electronegativity value more strongly attracts the bond’s electrons.
Across each period from left to right, there is an increase in electronegativity. Therefore, the alkali metals are the least electronegative elements, and the halogens are the most electronegative. The noble gases have been assigned electronegativities of zero.
Electronegativity decreases from top to bottom in each group. The decreasing electronegativity is a result of increasing atomic radii and decreasing ionization energy.
Electron affinity is the energy change that occurs when an electron is acquired by a neutral atom. As atoms add electrons, energy is released. This causes this process to be classified as exothermic, and the quantity of energy released is expressed by a negative number. Thus, elements with the largest negative electron affinity values accept electrons the most readily.
Reliable electron affinity values are difficult to obtain experimentally. Therefore, the electron affinity trends are not as clear or consistent as other trends that have been discussed. Generally, the electron affinity increases across the period from left to right with two notable exceptions. The alkaline earth metals and noble gases are least likely to gain electrons as indicated by their positive values.
Electron affinity generally decreases from top to bottom in each group with increasing atomic radii.
In conclusion, the instrument known as the periodic table is truly remarkable. The depth and scope of information able to be organized in it, as well as its flexibility of design, make it indispensable to any study in any field of chemistry.
Some transition elements have a variable valence or oxidation number because:
The correct answer is B. Most transition metals have one or two electrons in the outermost energy level and an incomplete d sublevel in the next to outermost level.
Elements in the same group of the periodic table have the same _______________
The correct answer is A. Elements in a group or family have the same number of electrons in the outermost energy level.