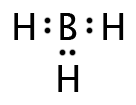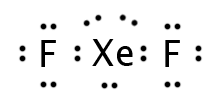In this lesson we will review how Valence Shell Electron Pair Repulsion (VSEPR) theory helps us determine the shapes of molecules.
VSEPR and the Octet Rule
VSEPR stands for Valence Shell Electron Pair Repulsion. Stripped down to its barest essentials, it says that because electron pairs are negative and like charges repel each other, the pairs of valence electrons that we see in a Lewis structure will push each other as far apart as possible in three-dimensional space. While there are nuances that come into play, we already have enough information to get started. We’ll begin by looking at the shapes that arise for molecules that obey the Octet Rule.
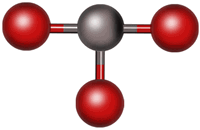
Consider carbon dioxide, CO2. Its Lewis structure is shown below. Although VSEPR contains the words “electron pair”, a more appropriate term might be “regions of electron density.” What difference does that make? Carbon dioxide has two C-O double bonds. Each double bond consists of two pairs of electrons, but for the purposes of VSEPR, it behaves the same way that a single pair of electrons (a single bond) would behave. For this reason, we will treat single, double and even triple bonds all the same way—as one region of electron density.
 Because there are only two regions of electron density around the central atom (carbon), they would move directly across the carbon atom from each other to get as far apart as possible. This is shown in the figure below. This shape is described as “linear” and the bond angle is 180º.
Because there are only two regions of electron density around the central atom (carbon), they would move directly across the carbon atom from each other to get as far apart as possible. This is shown in the figure below. This shape is described as “linear” and the bond angle is 180º.
 Boron trihydride, BH3, has a Lewis structure as shown below. Since there are only three regions of electron density, they will move 120º apart, forming a triangular pattern within a two-dimensional plane. This shape is described as “trigonal planar” with a bond angle of 120º.
Boron trihydride, BH3, has a Lewis structure as shown below. Since there are only three regions of electron density, they will move 120º apart, forming a triangular pattern within a two-dimensional plane. This shape is described as “trigonal planar” with a bond angle of 120º.
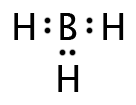
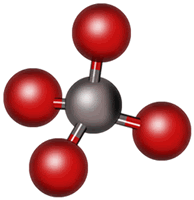
 Note the Lewis structure of methane, CH4, as seen below. There are now four regions of electron density. Our first impulse might be to expect them to move to the outer corners of a square, much like they appear in the Lewis structure. The problem is that this only utilizes two dimensions, whereas the molecule is able to use three dimensions. For this reason, the electron pairs actually move into the positions seen in the picture below. This shape is described as “tetrahedral” and the bond angle is just under 109.5°.
Note the Lewis structure of methane, CH4, as seen below. There are now four regions of electron density. Our first impulse might be to expect them to move to the outer corners of a square, much like they appear in the Lewis structure. The problem is that this only utilizes two dimensions, whereas the molecule is able to use three dimensions. For this reason, the electron pairs actually move into the positions seen in the picture below. This shape is described as “tetrahedral” and the bond angle is just under 109.5°.

 Let’s move on to ammonia, NH3. Looking at its Lewis structure below, we see four regions of electron density, just like methane; therefore, these four pairs of electrons will move apart into the tetrahedral pattern. There is an important difference between methane and ammonia, though. In ammonia, one of the regions is a lone pair; it has no atom attached to it on the other side. When we look at molecular shapes, even though it is the electron regions that repel each other, we only consider the atoms in the molecule. Looking only at the atoms, we see that the nitrogen atom is above the plane of the three hydrogen atoms, which are again in a triangular pattern. This shape is described as “trigonal pyramidal.” The bond angle is usually considered to be about 107°. The hydrogen atoms are slightly closer together than in methane due to the lone pair. Because there is not an atom on the other side, these unshared electrons are somewhat closer to the nucleus of the nitrogen atom; thus, they repel the other electron pairs slightly more.
Let’s move on to ammonia, NH3. Looking at its Lewis structure below, we see four regions of electron density, just like methane; therefore, these four pairs of electrons will move apart into the tetrahedral pattern. There is an important difference between methane and ammonia, though. In ammonia, one of the regions is a lone pair; it has no atom attached to it on the other side. When we look at molecular shapes, even though it is the electron regions that repel each other, we only consider the atoms in the molecule. Looking only at the atoms, we see that the nitrogen atom is above the plane of the three hydrogen atoms, which are again in a triangular pattern. This shape is described as “trigonal pyramidal.” The bond angle is usually considered to be about 107°. The hydrogen atoms are slightly closer together than in methane due to the lone pair. Because there is not an atom on the other side, these unshared electrons are somewhat closer to the nucleus of the nitrogen atom; thus, they repel the other electron pairs slightly more.

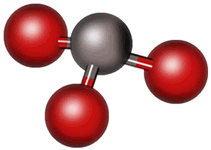 Finally, consider water, H2O. Its Lewis structure also shows four regions of electron density; therefore, the electron pairs will assume a tetrahedral pattern. But now there are two lone pairs, so we only see the central oxygen atom and the two hydrogen atoms off at an angle. Because of the bond angle (now around 105º due to the lone pairs), this shape is described as “bent.”
Finally, consider water, H2O. Its Lewis structure also shows four regions of electron density; therefore, the electron pairs will assume a tetrahedral pattern. But now there are two lone pairs, so we only see the central oxygen atom and the two hydrogen atoms off at an angle. Because of the bond angle (now around 105º due to the lone pairs), this shape is described as “bent.”


Multiple Central Atoms
So far, we have only dealt with small molecules—those with a single central atom. But many molecules are larger than this. How do we extend VSEPR to larger systems?
If we tried to create new names for shapes encountered in increasingly larger and larger molecules, we would quickly find ourselves with an extremely long and unwieldy list. VSEPR was meant to be simple and we shall keep it that way. In a molecule with more than one atom in the center, we will only consider one central atom at a time, using the shapes we have already learned to describe the geometry around that atom only.
Consider methylamine, CH3NH2. As we can see from its Lewis structure, it has two central atoms, carbon and nitrogen. Looking at the carbon first, we see four regions of electron density, all of which are bonded to atoms (three to hydrogen atoms and one to the nitrogen atom). The shape around the carbon must be tetrahedral. The nitrogen atom also has four regions around it, but one is a lone pair. The shape around nitrogen must be trigonal pyramidal.

VSEPR: Beyond the Octet Rule
As we encounter molecules that have more than eight electrons around the central atoms, we need new shapes to describe them. Fortunately, there are only two fundamental shapes to learn, corresponding to five and six regions of electron density. Unfortunately, each of these has multiple variations dependent upon lone pairs.
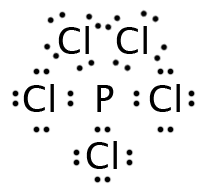
 Let’s start with a molecule whose Lewis structure we have seen before: PCl5. Five regions of electron density are around the phosphorus. Electrostatic repulsion pushes these regions into an interesting shape. Three of the electron pairs assume a triangular pattern, 120° apart from each other and in the same plane as the phosphorus. The atoms attached to these electron pairs are called equatorial atoms. The other two electron pairs align themselves along an axis that is perpendicular to the plane of the equatorial atoms. They are 180° apart from each other, but only 90º apart from the equatorial atoms. The atoms attached to these pairs are the axial atoms. The overall shape is described as “trigonal bipyramidal.” An example of see-saw is SF4.
Let’s start with a molecule whose Lewis structure we have seen before: PCl5. Five regions of electron density are around the phosphorus. Electrostatic repulsion pushes these regions into an interesting shape. Three of the electron pairs assume a triangular pattern, 120° apart from each other and in the same plane as the phosphorus. The atoms attached to these electron pairs are called equatorial atoms. The other two electron pairs align themselves along an axis that is perpendicular to the plane of the equatorial atoms. They are 180° apart from each other, but only 90º apart from the equatorial atoms. The atoms attached to these pairs are the axial atoms. The overall shape is described as “trigonal bipyramidal.” An example of see-saw is SF4.

 Let’s now consider ClF3. It, too, has five regions of electron density, three of which are bonding and two of which are lone pairs. The lone pairs will again occupy equatorial positions, creating a molecule that is “T-shaped.”
Let’s now consider ClF3. It, too, has five regions of electron density, three of which are bonding and two of which are lone pairs. The lone pairs will again occupy equatorial positions, creating a molecule that is “T-shaped.”

 Our last molecule with five regions of electron density is XeF2. Only two regions are bonding; the other three are lone pairs, which occupy all of the equatorial positions. This leaves only the central atom and two axial atoms visible, so the molecule is “linear.”
Our last molecule with five regions of electron density is XeF2. Only two regions are bonding; the other three are lone pairs, which occupy all of the equatorial positions. This leaves only the central atom and two axial atoms visible, so the molecule is “linear.”
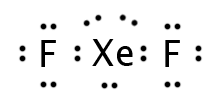
 Let’s move on to SF6. Its Lewis structure has six regions of electron density, all of which are bonded to a fluorine atom. The electron regions may achieve a separation of 90º from each other if they align themselves along the x, y and z axes, forming a shape we call “octahedral.” Don’t let the picture fool you; unlike the trigonal bipyramidal shape, there are no equatorial or axial positions. All six positions are equivalent.
Let’s move on to SF6. Its Lewis structure has six regions of electron density, all of which are bonded to a fluorine atom. The electron regions may achieve a separation of 90º from each other if they align themselves along the x, y and z axes, forming a shape we call “octahedral.” Don’t let the picture fool you; unlike the trigonal bipyramidal shape, there are no equatorial or axial positions. All six positions are equivalent.
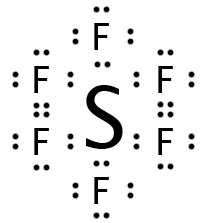

Our next molecule is BrF5. It has six regions of electron density, five of which are bonding and one of which is a lone pair. Since all six positions on the octahedron are equivalent, it does not matter which one is occupied by the lone pair, but it may be easier to visualize if we assume that it is the bottom position, creating a molecule that is “square pyramidal.”
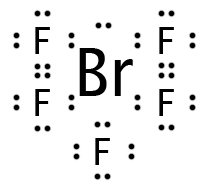
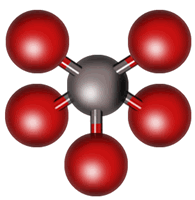
Our final molecule is XeF4 with six regions of electron density. Four regions are bonding; the other two are lone pairs, which we would expect to occupy positions across the central atom from each other. This leaves the central atom and four atoms in the shape of a square visible, so the molecule is “square planar.”

Question 1
Which of the following is the correct shape of the TeF4 molecule?
- Tetrahedral
- See-saw
- Square pyramidal
- Square planar
Reveal Answer
The correct answer is B. The Lewis structure shows five regions of electron density, one of which is a lone pair. This is typical of a see-saw structure.
Question 2
Which of the following is the correct shape around
either carbon in C2H4?
- Tetrahedral
- Trigonal planar
- Trigonal pyramidal
- Trigonal bipyramidal
Reveal Answer
The correct answer is B. The Lewis structure around either carbon shows three regions of electron density (Remember that the double bond only counts as one region!). This is typical of a trigonal planar structure.
Question 3
Which of the following is the correct shape of an ICl3 molecule?
- Trigonal planar
- Trigonal pyramidal
- T-shaped
- See-saw
Reveal Answer
The correct answer is C. The Lewis structure shows five regions of electron density, two of which are lone pairs. This is typical of a T-shaped structure.
 username@email.com
username@email.com