 username@email.com
username@email.com
In this lesson, we will review how oxidation numbers are assigned. We will also review how to write chemical names and formulas for molecular compounds, ionic compounds, and acids.
There are situations in which an atom does not have a clear and unmistakable charge, yet it would be helpful to determine whether or not it has lost or gained electrons. This is particularly true when dealing with oxidation-reduction reactions. In these situations, we need oxidation numbers. By convention in this lesson, oxidation numbers are written with the sign given first, followed by the number. Charges are given in the reverse order. Thus, “+2” is an oxidation number, while “2+” is a charge.
Oxidation numbers may be assigned to individual atoms by following these simple rules:
Rule 1 is straightforward. Fe, N2, and As are all examples of species whose oxidation numbers equal zero because they are elemental. Please remember that species such as Fe2+ or N3− are not elements but ions; thus, they do not fall under this rule.
Rules 2 and 3 are also rather simple. In the water molecule (H2O), each hydrogen atom has an oxidation number of +1 and the oxygen atom is −2. In sulfurous acid (H2SO3), each hydrogen atom is still +1 and each oxygen atom is still −2.
Rule 4 is easy when ions are written separately. The Cr3+ ion has an oxidation number of +3 while the Cl1− ion is −1. The use of this rule is less obvious when the ions are in a compound. For example, potassium sulfide (K2S) consists of a potassium ion, K+, and a sulfide ion, S2−. We do not see these charges in the compound’s formula, so we must infer them. Once we do, we can see that the oxidation numbers are +1 and −2, respectively.
Rule 5 requires the most thought. We invoke this rule when none of the others apply. Let’s reconsider H2SO3. The sulfur atom is not a simple sulfide ion here; rather, it is part of the sulfite ion, SO32−. As such, we cannot assign it a −2 oxidation number. Instead, we remember that each hydrogen is +1 and each oxygen is −2. In addition, bear in mind that the compound as a whole has no charge. Thus, all oxidation numbers must add up to zero. Representing sulfur’s unknown oxidation number with the variable “x”, we may find it with the following expression:

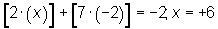
Which of the following gives the correct oxidation number for chlorine in calcium hypochlorite, Ca(ClO)2
The correct answer is B. Calcium is a monatomic ion (Ca2+) so its oxidation number is +2. Each oxygen is −2. The compound has no net charge so all oxidation numbers must add up to zero.
Which of the following compounds has the highest boiling point?
The correct answer is B. Calcium is a monatomic ion (Ca2+) so its oxidation number is +2. Each oxygen is −2. The compound has no net charge so all oxidation numbers must add up to zero. Chlorine is assigned as follows:
Which of the following correctly assigns the oxidation number to iron in Fe2(CO3)3?
Choice A is correct. The iron exists as a monatomic ion in this compound; however, iron may only have a charge of 2+ or 3+. This eliminates choices C and D. Because the carbonate ion has a 2− charge and there are three of them, each of the two iron ions must have a 3+ charge for the compound to be electrically neutral. This means that the oxidation number is +3.
In which of the following compounds does hydrogen not have an oxidation number of +1?
Choice C is correct. In a metal hydride, hydrogen is a monatomic ion with a charge of 1−, therefore, its oxidation number is equal to the ion charge, −1.
We recall that molecular compounds are formed when nonmetal atoms share valence electrons, frequently to achieve eight valence electrons for each atom. Often, the same two nonmetal atoms may be able to combine in more than one ratio, thus forming more than one potential compound. For example, sulfur is known to combine with oxygen in a 1:2 ratio (SO2) and in a 1:3 ratio (SO3). Since multiple combinations are possible, it is important that the name of a molecular compound indicates how many of each atom is present in that compound. This is done with prefixes. Below are listed the prefixes used in naming molecular compounds, along with the numerical value that each prefix represents.
| Prefix | Number | Prefix | Number |
|---|---|---|---|
| mono | one | hexa | six |
| di | two | hepta | seven |
| tri | three | octa | eight |
| tetra | four | nona | nine |
| penta | five | deca | ten |
The name of a binary molecular compound follows a straightforward pattern. The first element’s name is listed first, preceded by whichever prefix indicates how many of that element’s atoms are in the compound. The only exception to this is if there is only one of that atom. In that case, the prefix mono is omitted. The name of the second element follows, modified so that it ends in –ide. It is always preceded by the appropriate prefix. A few examples are listed below:
Which of the following correctly names the compound XeF4?
The correct answer is C. Because there is only one xenon atom, the prefix mono is omitted, making choices A and B incorrect. The name of the second element in the compound must be modified so that it ends with –ide, which was not done in choice D.
In ionic compounds, positive ions combine with negative ions in a ratio such that their charges add up to zero. This means that a particular pair of ions will always combine in the same ratio, which makes the use of prefixes unnecessary. For this reason, when we name any ionic compound, we simply name the positive ion first and the negative ion second. When we are given that name, we must know the appropriate charge on each ion in order to put together a correct formula.
A binary ionic compound contains only monatomic ions. Positive monatomic ions have the same name as the element from which they come. Negative monatomic ions are named by modifying the name of the element so that it ends with –ide. Many of the elements in the s block and the p block of the Periodic Table follow the Octet Rule when bonding ionically, so their charges are fairly easy to predict. The table below summarizes these charges:
| Group | Charge |
|---|---|
| Group 1A | 1+ |
| Group 2A | 2+ |
| Group 3A | 3+ |
| Group 4A | no ion formed |
| Group 5A | 3- |
| Group 6A | 2- |
| Group 7A | 1- |
| Group 8A | no ion formed |
Armed with this information, we should be able to write names and formulas for many simple binary ionic compounds. For example, a compound made from calcium and bromine would contain Ca2+ ions and Br1− ions. We would name this calcium bromide. To make the net charge of the compound zero, we would need two bromide ions for every calcium ion, so the formula would be CaBr2
Which of the following gives the correct name and formula for a compound made of sodium and phosphorus?
The correct answer is C. Choices B and D have an incorrect formula. Three Na+ ions are needed for every P3− .ion in order for the charges to add up to zero. Choices A and C both have the correct formula, but choice A uses the prefix tri, which is incorrect for an ionic compound.
Many transition metals, as well as several metals near the bottom of the p block (most commonly Sn and Pb), are able to form at least two different ions. Iron, for example, can form Fe2+ and Fe3+. If we were to name a compound iron sulfide, we would have no idea which ion was in the compound in question. This would then prevent us from being able to write the formula.
We solve this dilemma by using Roman numerals in parentheses as part of the ion’s name. Thus, the name of Fe2+ is not just iron, but iron(II). Fe3+ is named iron(III). Iron(II) sulfide is made from Fe2+ and S2− ions, so its formula is FeS. Iron(III) sulfide contains Fe3+ and S2− ions, so its formula is Fe2S3.
Which of the following is a correct pairing of a name and formula?
Answer The correct answer is D. The tin(II) ion is Sn2+and the chloride ion is Cl1−. One Sn2+ is needed for every two Cl1− ions, so the correct formula is SnCl2. The correct formula for tin(IV) chloride would be SnCl4, which does not appear in either choice A or B.
Many ionic compounds contain polyatomic ions. These compounds follow the same rules that we have seen before, with only a few minor differences. Most polyatomic ions are negative (ammonium, NH4+, is the most common exception), but their names do not usually end with –ide. Most polyatomic ions have names that end with –ite or –ate. Sulfide is simply a sulfur atom with a charge—S2−. Sulfate, on the other hand, is a sulfur atom and several other atoms together that have a charge—SO42−.
The most common polyatomic ions contain an atom along with one or more oxygen atoms. The ion whose name is the element name modified so that it ends in –ate will be considered our “base” ion. For example, if we modify “nitrogen” so that it ends with –ate, we get “nitrate.” So the nitrate ion, NO31−, will be considered our base ion. Unfortunately, there is no hard and fast rule regarding how many oxygen atoms are in the base ion, although it is usually three or four. Once the base ion is known, however, there is a pattern to the names of the other ions made with that element. The following table shows this pattern and uses chlorine (base ion of chlorate, ClO31−) as an example.
| base ion minus two oxygens | hypo—root—ite | hypochlorite, ClO1− |
| base ion minus one oxygen | root—ite | chlorite, ClO21− |
| base ion | root—ate | chlorate, ClO31− |
| base ion plus one oxygen | per—root—ate | perchlorate, ClO41− |
By following this pattern, we should be able to see that since NO31− is the nitrate ion, then NO21− is the nitrite ion.
The only other difference when using polyatomic ions concerns the use of subscripts in the compound’s formula. When a formula requires more than one of a polyatomic ion, we must put the ion formula in parentheses and place the subscript outside of the closing parenthesis. For example, calcium nitrate requires one Ca2+ ion and two NO31− ions; therefore, its formula is Ca(NO3)2.
Which of the following is the correct formula for copper(I) sulfate?
The correct answer is A. The correct formula for the sulfate ion is SO42–, ruling out choices B (copper(I) sulfite) and D (copper(I) sulfide). Choice C does not have a ratio of ions whose charges add up to zero. Since copper(I) is Cu+, the correct formula is Cu2SO4.
Which of the following is the correct name for SF6?
Choice B is correct. Sulfur and fluorine are nonmetals that form a molecular compound, so we must use prefixes in the name, eliminating choice A. Choice C is incorrect because fluorine has not been modified to end with –ide. Choice D is incorrect for the same reason and because the prefix mono should be omitted as there is only one sulfur atom in the molecule.
Which of the following is the correct name for MnO2?
Choice D is correct. Like most transition metals, manganese can have several different charges; therefore, the name must use a Roman numeral to tell which ion is present in this compound. Only choice D uses a Roman numeral.
Which of the following is the correct name for Ca(ClO)2?
Choice C is correct. Choice A is incorrect because this compound is ionic, so prefixes should not be used. ClO1– is a polyatomic ion so it does not end with –ide, ruling out choice B. ClO1– is not the base ion so it cannot end with –ate, ruling out choice D. Because it has two oxygen atoms less than the base ion, it should begin with hypo- and end with –ite, as in choice C.