 username@email.com
username@email.com
In this lesson, we are going to cover the important features of our Earth.
The Earth, like many celestial bodies, is not a uniform solid object; it has a series of layers of varying densities and characteristics. Our planet is made up of many constituent elements and compounds that are responsible for the components and physical properties of the planet we call home. The main layers are:
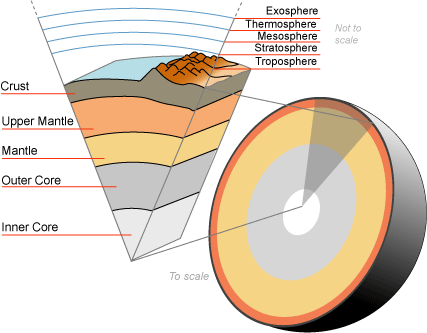
Describe the constituency of the Earth.
The correct answer is C. We know from seismic wave, microwave, and sonar analyses, that the Earth is not a uniform solid, but an interactive series of layers with different properties and materials.
The surface of the earth is covered by what is labeled the crust. This is divided into two types: the continental and oceanic crusts. Because the density of the water is less than the density of the continent, the water floats on top of the continental crust. The oceanic crust is crust covered by ocean by virtue of its elevation. The oceanic crust is a little denser than continental crust as well as much thinner.
The continental crust is formed as volcanic magma cools on the surface. Most of this volcanic activity is found in subduction zones where one plate is being pushed under another plate. As this liquid rock erupts, it spreads and repeatedly covers the surface in layers, and more continental crust is built up. Over time, particular materials are carried by the wind or water and deposited on the surface along with seeds dropped by birds or carried by the wind or water. With time, plants grow and stabilize the surface.
In areas where there is little stabilization from living inhabitants, weathering processes occur, including temperature changes that expand and contract the rocks, and rain which can freeze and crack the rocks or dissolve more soluble materials to expose and weaken the overall structure of the rocks. Eventually, over long periods of exposure, much of the rocks are either dissolved or deposited by erosion into the rivers and streams. These kinetically active areas spread the weathered materials to lower points of elevation and form such areas as fertile river deltas, rich in sediments that build up over time.
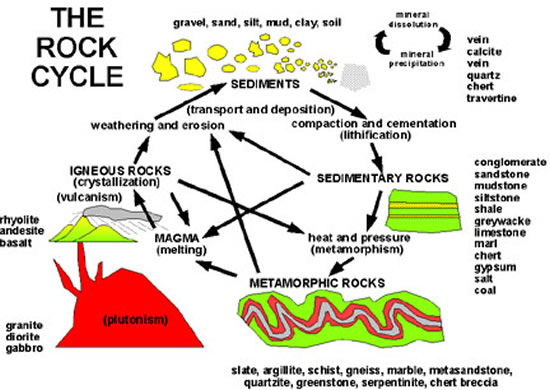
For a very long time, geologists examined rock and sediment layers on the sides of hills, in deep canyons, or on the sides of mountains all over the world. Upon inspection, it is easy to assign a relative order to the geologic events that laid down these layers. The sandstone on the bottom came first, then later the layer of limestone, and much later the clay layer, and so on. The basic concepts of a geologic time scale began late in the 17th century when scientists correctly deduced that each rock strata represented a different time period.
During the 18th century, scientists realized that they could not read rock strata as simple layers because geological processes deep in the earth complicate the layer order. Strata once on the ocean floor millions of years ago could now be on the top of a mountain. It can be distorted, eroded, or tilted long after it was deposited. Rock strata deposited at the same time in different parts of the world can vary in composition greatly. Clearly, marking distant geologic times by types of rock strata in layers only gives part of the picture.
By the 19th century, geologists had begun to classify strata according to the types of fossils found within them. This made it possible to identify similar strata on different continents if they contained the same kinds of fossils. These observations led to the development of the relative geologic time scale. However, the actual age of these rock strata and the fossils they contained remained a topic of heated debate for many decades. It wasn’t until after the discovery of radioactivity, in the late 1890s, that a better method would be developed to determine the age of rocks, fossils, and other material found in any rock strata. This tool would give geologists what was needed to date rock strata and to develop a chronology of the Earth’s history.
Geologists have known for some time that the entire history of the Earth is preserved in the strata. There you can easily see the clues to the geological and biological processes of ancient times. The geologic time scale displayed below was developed over a long period of time and was most recently updated in 2004 by the International Commission on Stratigraphy.
| Eon | Era | Period | Ended MYA* |
|---|---|---|---|
| Phanerozoic | Cenozoic | Neogene | Now |
| Paleogene | 23 | ||
| Mesozoic | Cretaceous | 65 | |
| Jurassic | 140 | ||
| Triassic | 205 | ||
| Paleozoic | Permian | 245 | |
| Pennsylvanian | 290 | ||
| Mississippian | 325 | ||
| Devonian | 355 | ||
| Silurian | 415 | ||
| Ordovician | 440 | ||
| Cambrian | 495 | ||
| Proterozoic | Neoproterozoic | Ediacaran | 542 |
| Cryogenian | 630 | ||
| Tonian | 850 | ||
| Mesoproterozoic | Stenian | 1000 | |
| Ectasian | 1200 | ||
| Calymmian | 1400 | ||
| Paleoproterozoic | Statherian | 1600 | |
| Orosirian | 1800 | ||
| Rhyacian | 2050 | ||
| Siderian | 2300 | ||
| Archean | Neoarchean | 2500 | |
| Mesoarchean | 2800 | ||
| Paleoarchean | 3200 | ||
| Eoarchean | 3200 | ||
| Hadean | 3800 | ||
*Millions of years ago
The accepted age of the Earth (and the rest of our solar system) is about 4.6 billion years. This age is computed through a number of different methods. Unfortunately, the age of the Earth cannot be determined from terrestrial rocks since they have not been able to withstand the effects of such a volatile planet.
The Earth is a vibrant and thriving planet whose surface is completely reworked through the process of plate tectonics every 500 million years or so. New rock structures are constantly being forged and brought to the surface through our planet’s dynamic geological machine. That’s why it’s impossible to find rocks on the Earth’s surface that date back 4.5 billion years. The processes of weathering and erosion compound this problem.
The most common method for determining the age of rocks is radioactive dating. Radioactive isotopes change into stable isotopes through the process of radioactive decay. This process changes the total number of protons in the nucleus and thus changes the isotope through time. This is commonly referred to as an object’s isotopic signature. The time it takes for half of the radioactive materials in a sample to decay into another isotope is called the half-life. Carbon-14 is a well-known isotope that has a half-life of 5730 years.
Carbon-14 dating is very useful for archeologists wanting to date an ancient artifact or to paleontologists wanting to determine the age of a fossil. Over time, carbon-14 decays into nitrogen-14. The amount of carbon-14 that is left in a sample is an indication of that sample’s age. Although carbon-14 can be used to determine the age of materials up to 40,000 years old, this is too short a time span to be useful in determining the age of the Earth.
This table lists some of the many radioactive isotopes with half-lives longer than that of carbon-14.
| Radioactive Isotope | Decay Product | Half-Life |
|---|---|---|
| Uranium-238 | Lead-206 | 4.5 billions years |
| Uranium-235 | Lead-207 | 704 million years |
| Thorium-232 | Lead-208 | 14 billion years |
| Rubidium-87 | Strontium-87 | 48.8 billion years |
| Potassium-40 | Argon-40 | 1.25 billion years |