 username@email.com
username@email.com
The focus of this lesson is to discuss the theory of the formation of our atmosphere.
The air in our atmosphere is a mixture of different gases
Nitrogen, oxygen, argon, and carbon dioxide combine to make up 99.998% of the volume of the atmosphere.
Atmospheric water vapor is replenished constantly by the water cycle.
The water cycle also delivers heat energy to the atmosphere.
Our atmosphere evolved over a period of billions of years. The atmosphere we have today is very different from the atmosphere of the very young Earth. That atmosphere would be as alien to us as the atmosphere of another planet. In fact, it is by studying the atmospheres of other worlds in the solar system that we have a good idea about the evolution of our atmosphere. The geological, chemical, and biological processes that shaped our planet controlled this evolution.
4.6 billion years ago, the early Earth was surrounded by an atmosphere of mostly hydrogen and helium. These gases came from the cloud of material from which the solar system formed. Given the Earth’s distance from the newly forming sun, this atmosphere may have also contained numerous hydrogen compounds such as ammonia, methane, and water vapor. This atmosphere was not destined to stick around very long. Hydrogen and helium are lightweight gases. These gases were gradually lost to space because the gravitational pull of the Earth was too weak to hold an atmosphere made of hydrogen and helium. This early atmosphere was depleted within the first 100 million years. However, during this time, processes were already at work that would lead to the next atmosphere.
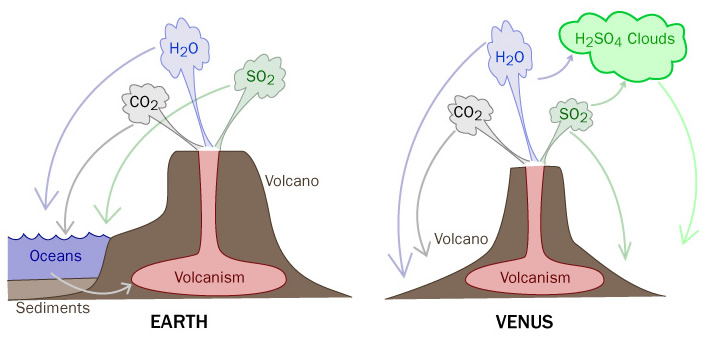
During the formation of our planet, many different gases were delivered to our world through collisions with comets and asteroids. These gases became trapped inside our planet and were eventually released through geological processes such as volcanoes and steam vents. The gases vented through these processes are listed in the table below.
| Volcanic Gas vented on Early Earth | Percentage by Volume |
|---|---|
| Water Vapor (H2O) | 79% |
| Carbon Dioxide (CO2) | 12% |
| Sulfur Dioxide (SO2) | 7% |
| Nitrogen (N2) | 1% |
| Hydrogen (H2) | 0.5% |
The large amount of water vapor that was vented eventually fell back to the surface. This water collected on the surface and became the first rivers, lakes, and oceans. In the previous lesson, we learned that water and carbon dioxide are greenhouse gases. So we can assume that the global climate was much warmer at this time that it is today. A good model of this early atmosphere is the planet Venus. The atmosphere of Venus is composed of 96% carbon dioxide. This abundance of a greenhouse gas contributes to Venus’ surface temperature of 485°C (almost 900°F!).
The same volcanic processes that helped to shape Earth’s early atmosphere were also at work on Venus with one exception. Venus did not receive the abundance of water that the Earth received. The presence of bodies of water on the surface of the Earth became instrumental in absorbing much of the carbon dioxide from the atmosphere. This trapped carbon dioxide eventually became bound into the ocean sediments and over billions of years became locked into carbonate sedimentary rocks such as limestone. Over time, some of this ancient carbon is released back into the atmosphere as carbon dioxide through volcanism where it is once again absorbed by the oceans and other processes.
Our atmosphere today is rich in oxygen. Oxygen is a very important gas that sustains animal life on this planet. However, the early atmospheres of Earth did not have much oxygen. The story of how oxygen levels increase in our atmosphere is a story of sunlight and plant life. All of the oxygen in the early atmosphere was bound up in the molecules of carbon dioxide (CO2) and water (H2O).
Long before there was a protective ozone layer, strong ultraviolet light pierced the atmosphere all the way to the surface. The energy of this ultraviolet light was absorbed by carbon dioxide and water. These molecules were broken apart by photolysis. Carbon dioxide was broken down to carbon monoxide and atomic oxygen while water was broken down to atomic hydrogen and a hydroxyl. This atom of hydrogen would escape into space.
CO2 → CO + O
H2O → H + OH
The atomic oxygen from the breakdown of carbon dioxide would eventually interact with the hydroxyl from the breakdown of water. This reaction resulted in atomic hydrogen and molecular oxygen. The hydrogen from this reaction would also escape into space leaving behind oxygen gas.
O + OH– → O2 + H+
Photolysis was a slow process that probably only yielded about 1% of the atmospheric oxygen. The abundance of oxygen we have today in our atmosphere was made possible by plant photosynthesis. However, before photosynthesis could happen, primitive life had to be protected from ultraviolet light. The process of photolysis increased the levels of oxygen in the upper atmosphere. Sunlight there acted upon on it to form ozone. This ozone layer became a protective shield that blocked most of the harmful ultraviolet radiation from the sun. This made it possible for prokaryotic life such as cyanobacteria to develop photosynthesis as a metabolic process. This primitive photosynthesis contributed to an increase in atmospheric oxygen that made it possible about 2 billion years later for eukaryotic life to evolve.
Photosynthesis in eukaryotes was much more efficient and oxygen levels continued to climb. However, much of this oxygen was robbed from the atmosphere by the abundance of dissolved iron in the ocean. This iron easily bonded to oxygen removing it from the atmosphere and storing it in red sedimentary layers. Once the majority of the iron was removed from the oceans through this process, atmospheric oxygen began once more to steadily increase. About 400 million years ago, vascular plant life began to inhabit dry land. These plants, and their descendants, contributed the majority of the oxygen in the atmosphere. Today, plant photosynthesis is responsible for maintaining the atmospheric oxygen concentration of 20%. Without the process of photosynthesis over billions of years, we would not have the oxygen we need to breath.