 username@email.com
username@email.com
In this lesson, we’ll study how wave energy transfers through or across a material.
Energy has the capability of producing change, and that energy can be transferred from one location or form to another without losing any energy.
Drop a pebble in a lake and waves spread out in all directions. Clap your hands and sound waves move throughout the air around you. Shake the end of a taut rope and a wave pulse moves down the rope. In each case, a disturbance produces waves, and this disturbance moves through or across a material (water, air, and the rope in our examples). The material through which the wave travels is called the medium.
What exactly is a wave? A wave is disturbance that carries energy and moves though a medium without producing a net change in the medium. But in what way does this wave move through the medium? When waves hit a bobber floating in the water, for example, the bobber simply “bobs” up and down (as well as forward and backward). So the bobber (which is part of the medium) vibrates as the wave energy goes through it. Likewise, in the case of the wave moving down the rope, the rope also vibrates as the wave moves through it. In other words, the medium does not follow along with the wave. As a rule, wave energy propagates through a medium without any gross displacement of the medium itself.
Vibrations of physical objects disturb air and produce sound waves. A guitar string, for example, vibrates the surfaces in the guitar. These surfaces push back and forth on the air and sound waves spread out into the room. The particles of air between the source and the receiver vibrate back and forth as the sound energy travels through them. If the air vibrates back and forth 256 times per second, the receiver hears the note “C.” The more intensely the air vibrates, the louder the note is perceived. Sound can also travel through other media like water and steel.
As the wave pulse travels to the right in the rope pictured, what happens to the section of rope marked “X?”
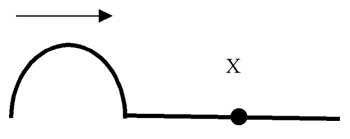
The correct answer is B. The medium simply vibrates as the wave energy travels through it. With waves, there is never any gross displacement of the medium.
In the last lesson, we reviewed the fact that a medium vibrates as wave energy travels through it. Depending on the type of wave, the medium may vibrate in different ways relative to the motion of the wave.
If the medium vibrates up and down at 90-degree angles to the motion of the wave, the wave is classified as a transverse wave. For example, the point “X” on the rope pictured below first travels up and then down as the wave crest travels to the right.
Gas pressure is the result of the collision of gas particles with an object. What would happen to the pressure if the number of particles in a certain volume were increased? Kinetic theory of ideal gases predicts the there will be more collisions between the particles and thus more pressure. Besides depending on the number of particles, pressure also depends on the average kinetic energy of the particles, or temperature.
What if different gases are combined at a constant volume and temperature? At constant temperature, the average kinetic energy of the mixture is constant and the type of gas particle is not important. Each gas will contribute to the new pressure as it is combined with the mixture. The contribution of each gas in the mixture makes to the total pressure is called the partial pressure. John Dalton (1766-1844) proposed that in a mixture of gases, the total pressure is simply the sum of all the partial pressures in the gases. This is called the law of partial pressures.
For example, consider two gas containers. One is filled with oxygen gas at a pressure of 300 kPa, another is filled with nitrogen gas at a pressure of 400 kPa, and both containers are at the same temperature of 25 ° C. If both gases are combined in the same container, what is the pressure of the combined gases?
According to the law of pressures we add the pressure of the oxygen (P 1) to the pressure of the nitrogen (P 2) as follows:
P total = P 1 + P 2 = 400kPa + 300kPa = 700kPa