username@email.com
username@email.com
In this lesson, we are going to cover the main aspects of nuclear fission and fusion, including isotopic and radioactive concepts.
We reviewed the chemical properties of solutions in the last lesson, including the chemistry of electrochemical cells (aka batteries).
All of the chemical reactions that we have discussed have involved only the exchange of electrons on the outside of the nucleus. Elements with the same number of protons but different number of neutrons (such as tritium, deuterium, and protium — are all forms of hydrogen having one proton and three, two, and one neutron respectively) and are called isotopes. Sometimes elements with unstable nuclei (due to larger numbers of protons and neutrons) can lose nuclear particles that change the number of either protons or neutrons in the nucleus. These are called radioactive elements because a large amount of energy is usually released as the nucleus loses mass. As the nucleus decays, the atoms of one element are transformed into the atoms of another element. This is called natural transmutation. For elements greater than number 83, this produces isotopes that are radioactive, also known as radioisotopes.
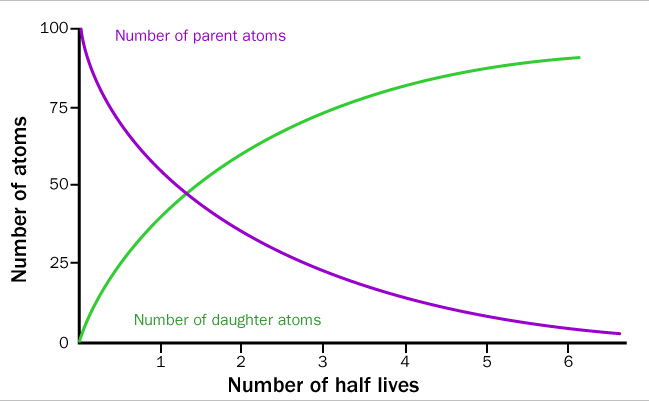
Radioactive elements follow a predictable rate of decay based on their known half-life. These reactions are similar mathematically to the rate of reaction discussed earlier. As the parent atoms decrease, the daughter atoms increase proportionally.