 username@email.com
username@email.com
We are now going to review the important components at the juncture between physics and chemistry on a macro scale. These include the concepts of motion, collisions, temperature, and pressure. At the end of this lesson, we will have a better understanding of how the physical properties of a system affect chemical properties and reactions.
We have now reviewed the four different kinds of reactions and how to balance a chemical equation. We covered all the material we needed to know in order to identify and describe any reaction and effectively balance both sides of a chemical reaction.
All particles of matter are in constant random motion because of the energy present in the environment on Earth. We associate this energy with temperature, an average measure of the kinetic energy of particles. When the temperature increases, the average kinetic energy increases, and the amount of random motion also increases. This seems apparent when we observe steam billowing out of a boiling pot of water, or the movement of smoke directly over a fire. Similar to the way that energy is translated when one pool ball hits another pool ball, atomic sized particles also collide with each other and translate that motion between and among particles. When a particle hits the bottom of a thermometer, that movement is transferred to the atoms of mercury in the tube. More collisions mean that the mercury rises higher. The height of the mercury is a way for us to have a relative idea of how many particles are banging against the bulb of the thermometer.
Cold is not the opposite of heat; it is the absence of heat, or the reduction in the amount of random motion. Only at absolute zero (0 Kelvin, -273 ° Celsius, or -459.67° Fahrenheit ) does motion theoretically come to a complete stop (Note: Kelvin is not measured in degrees). This is the temperature where there is zero kinetic energy. Since this is colder than any place found naturally on Earth, it is beyond the range of common experience and probably only exists theoretically or in the farthest reaches of the universe. We could never measure absolute zero simply because our presence (or instrumentation) would make the energy of the system greater than zero.
Particles in motion move from areas of higher concentration to areas of lower concentration. This is called diffusion and is part of the idea of entropy. This can be observed when a candle is lit and the aroma slowly moves across the room. Eventually, the concentration of all molecules will become equally distributed, and a sample of air in a closed space will be the same no matter which part of the space was sampled. The rate at which this occurs depends on the temperature of the space. At higher temperatures, this equilibrium occurs more rapidly due to the increased speed of the molecules.
The first law of thermodynamics states that the energy of the universe is constant. Energy is neither created nor lost but it can change form. Kinetic energy is the energy of motion that can be observed in physical states of matter. In solids, vibrational energy exists, but the particles are not free to completely move past each other, due to the potential energy stored in the bonds holding the atoms together. In liquids, the particles are free to flow past each other and are at a higher kinetic energetic state than in solids. In a gas, the particles are moving so rapidly that the atoms are many thousands of times more distant from each other than in a liquid.
Temperature is a measure of the average speed of the particles measured in degrees. Heat is a measure of the transfer of energy required to increase the temperature of a given substance, and depends on the amount and characteristics of the substance in question. Heat is measured in units called calories. When a substance changes state, it will release or absorb energy, but will not change temperature. Although this seems a bit counterintuitive, it makes sense. Realize that it takes energy to change state (e.g., from liquid to gas) and that while more heat may be applied, the energy is used to change state, not increase temperature. Boiling water at sea level is always going to be 100 ° C; never more, never less. The energy required to change states is called latent heat energy. This can be observed as an ice cube melts. The temperature of the ice cube will remain constant (near 0 ° Celsius) but the ice will turn to water because heat energy is giving the molecules energy to break free from the fixed position. Heat always flows from where it is to where it is not. This is the fundamental phenomenon that drives everything from volcanoes to hurricanes.
Any time a new substance is formed, when the kinetic energy of collisions activates the potential energy of the substance, bonds may be broken or formed. This formation of bonds occurs to form something more stable if energy is given up. Reactions that release energy are called exothermic. Examples of exothermic reactions include:
Endothermic reactions require energy to make bonds and usually result in a substance that is less stable. These are reactions that often feel cold when they happen because the products have more energy than the reactants, and need an input of energy, usually in the form of heat.
For example:
When the pressure increases, it pushes the particles closer together. When the temperature increases, energy increases, making more random motion available to move particles farther apart. Temperature and pressure can be varied to change many pure forms of matter into different states. Below is a graph that represents all three forms of matter at different temperatures and pressures.
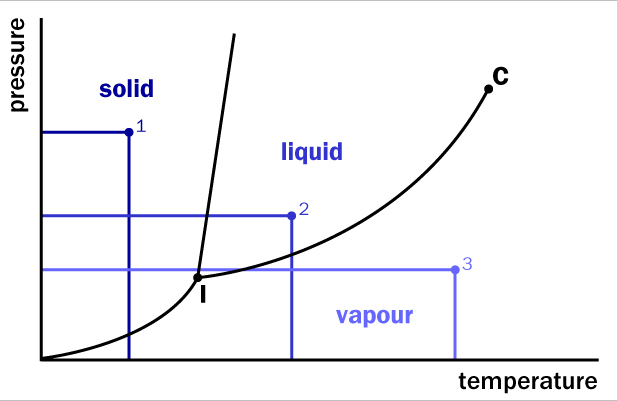
The triple point (labeled I) is the point where a substance can exist in all three states and is a unique thermodynamic equilibrium to each substance. For example, ice can evaporate (to become a gas) in your freezer without ever being a liquid. Leave an ice cube out to observe for a week in the freezer and you will see. Dry ice, (solid carbon dioxide) also evaporates to become a gas at room temperature. Skipping the liquid state is often called sublimation. The nature of the substance determines the temperature and pressure required for phase changes. The Critical point, (labeled C), denotes the temperature at which pressure cannot overcome the energy available to the particles and the substance cannot be compressed into a liquid at that temperature. The normal melting point is the temperature under normal conditions (one atmosphere of pressure) when a substance changes from a solid to a liquid. The normal boiling point is the temperature under normal conditions (one atmosphere of pressure) when a substance changes from a liquid to a gas.