 username@email.com
username@email.com
In this lesson, you will review proteins.
Proteins are the stuff of life. The genetic information found in DNA contains codes for production of proteins. As enzymes, they control metabolism and literally make life functions possible. They are involved in transport, immunity, and storage, and dynamically function as hormones. It is the shape of a protein that gives the protein its unique function. Its shape is determined by the chemical composition of the protein. We deconstruct protein shapes into secondary, tertiary, and quaternary structure, depending on complexity.
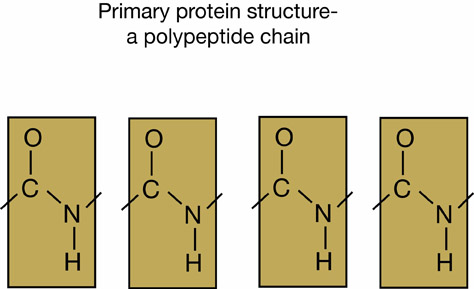
Examples of a polypeptide chain
Proteins are composed of amino acid monomers, which always have a nitrogen-based amine group and a carboxyl group bonded to a carbon or hydrocarbon side chain. The unique side chain of each amino acid is usually designated “R.” There are 20 unique amino acids. The R group of an amino acid affects its reactivity. Some amino acids have polar side chains, while others have non-polar side chains; some act as acids, others as bases. The sequence of amino acids in a primary protein determines its ultimate shape.
Amino acids undergo dehydration synthesis to form polypeptide bonds. Polypeptide chains represent the primary level of protein structure.
The secondary structure of a protein results from hydrogen bonding between amino acids in the peptide chain. This leads to twisting or folding of the chain into the alpha helix and the beta pleated-sheets based mostly upon weak hydrogen bonds between peptides.
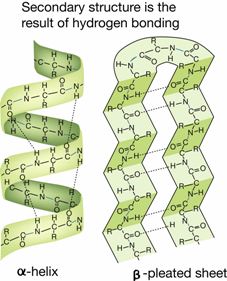
Example of secondary structure of a protein
Tertiary structure results from the hydrophobic effect—a folding of the polypeptide chain due to positioning of polar and non-polar amino acids. Non-polar amino acids are typically inside the three-dimensional structure and polar side chains are on the outer surface. Hydrogen bonds and disulfide bonds stabilize tertiary structure.
Quaternary structure involves a complex grouping of two or more polypeptide chains into a stable, multi-subunit structure. They are stabilized by hydrogen bonding, van der Walls interactions and ionic bonding, and occasionally by disulfide bonds. Rubisco, the most abundant protein on Earth, has 16 subunits. Not all proteins have quaternary structure, and some function as simple primary polypeptide chains.
Proteins are important structural elements, especially in animals. The proteins actin and myosin are the main components of the cytoskeleton. These proteins function in cell movement and support. Actin and myosin are the main component of muscle cells and their interaction results in muscle contraction. Much of the non-cellular matrix of our bodies is composed of protein.
Proteins are also important structural components of cell membranes. Proteins embedded in cell membranes allow the control of movement of materials in and out of cells. Many membrane-bound proteins act as enzymes.
Proteins function in transport as well. Hemoglobin, the protein found in red blood cells, transports oxygen around the body. Sickle cell anemia is a disease that is caused by a misshapen hemoglobin protein, making it less efficient at transporting oxygen. Lipoproteins are important in transport of lipids. Within cells, molecules are transported along proteinaceous cytoskeletal elements.
Many hormones are also proteins. Insulin and human growth hormone (HGH) are proteins that are now produced by genetically engineered bacteria for use in treating human disease. Protein hormones function by interacting with specific chemical receptors on cells. Hormone receptor reactions are called signal transduction pathways and depend on the physical fit between the molecules.
The immune system produces proteins called antibodies that neutralize pathogens, such as viruses. Antibodies function by fitting correctly with the invader it is designed to neutralize. Once a pathogen has been encountered, the immune system “remembers” how to produce the correctly shaped antibody.
Enzymes are proteins that control all of the chemical reactions in cells. They do so by acting as organic catalysts, lowering the activation energy needed for chemical reactions. Their action allows metabolic reactions to occur millions of times faster than they would proceed uncatalyzed. Enzymes are important parts of many metabolic pathways and are vital to both anabolic and catabolic reactions.
Enzymes catalyze reactions by binding with specific substrates. When enzyme-substrate complexes are formed, molecules of reactant substrates are positioned in a way that destabilizes their bonds, allowing chemical reactions to proceed. Such destabilization is called the transition state of a chemical reaction. A substrate is converted to a product and released by the enzyme. Enzymes are neither used up nor changed by the reactions they catalyze.
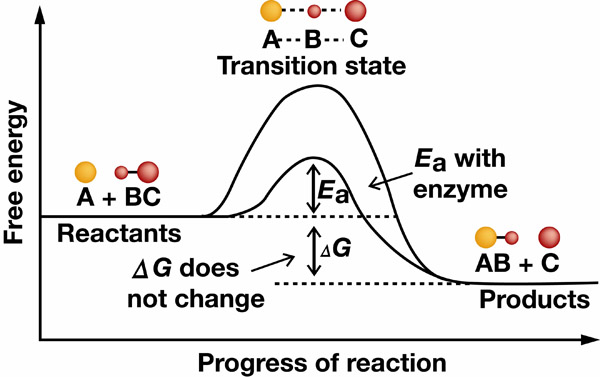
Example of how enzymes help reactions progress
Enzyme function depends on the physical fit with the substrate. Substrates bind at an enzyme active site in a key-in-lock or hand-in-glove configuration. Many genetic diseases in humans are caused by misshapen enzymes. It is possible for cells to control enzyme activity by altering the shape of the enzyme or by blocking the binding site.
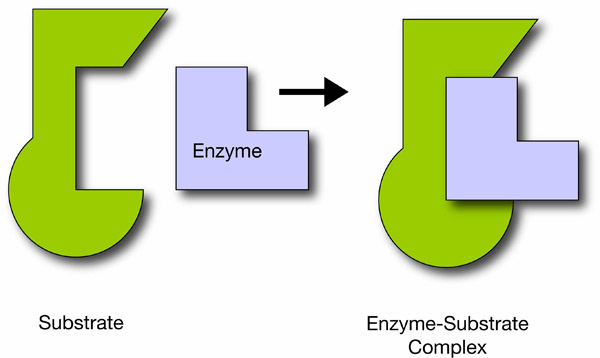
Enzymes’ lock-and-key substrate complex
Many enzymes need other molecules called cofactors in order to function. There are three kinds of these non-protein molecules: coenzymes, activators, and prosthetic groups. Coenzymes are organic molecules that usually serve to transport atoms or functional groups between enzymes. Many vitamins act as coenzymes, as do ATP and other nucleotides. One of the most important coenzymes is nicotinamide adenine dinucleotide (NAD+), which often acts as an electron acceptor in a process called enzyme-catalyzed oxidation-reduction. Activators are inorganic ions that are required for full enzyme activity. One of the most common examples is the calcium ion needed for blood clotting by the enzyme thrombokinase. Similarly, magnesium and potassium are also common activators for enzymes. Prosthetic groups are basically tightly bound cofactors and are usually responsible for transporting electrons for the enzyme.
Understanding enzyme function is vital in modern medicine and biotechnology. Much of our knowledge comes from the study of enzyme kinetics. The rate of enzyme catalyzation can be calculated by changes in the concentrations of reactants and product(s). Each specific enzyme complex has a rate constant (KM) derived from the substrate concentration, at which an enzyme-catalyzed reaction proceeds at half its maximum velocity. The Michaelis-Menten equation is used to describe the velocity of an enzyme-catalyzed reaction at unlimited substrate concentration. Enzyme function can be analyzed via comparison to kinetics of function under the Michaelis-Menten equation.
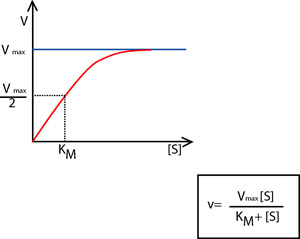
The Michaelis-Menten equation and graph showing the relationship
The rate of enzyme catalysis depends on temperature, pH, ionic concentration, the nature of the reactants, and the relative concentrations of enzymes and substrate(s). Many important enzymes are inhibited at very high temperatures due to denaturation or change in protein shape. Each enzyme has an optimal temperature range for highest activity.
Shifts in pH and ion concentration can also change the non-covalent bonding patterns and shapes of proteins. Recall that protein shape beyond the primary polypeptide is the result of reactions between amino acid side chains. Hydrogen and polar bonds are readily broken when pH changes. When the non-polar bonds break, secondary and tertiary enzyme structure can change, and the substrate may no longer fit the active site of the enzyme.
The relative amounts of enzyme and substrate also affect the rate of activity. When all of the active enzyme sites become bound to substrate, the system is saturated. That means the chemical reaction being catalyzed is proceeding at the maximum rate and adding more substrate will not change that rate. The Michaelis-Menten equation was derived based on conditions of substrate saturation.
Often the products of enzyme-catalyzed reactions will serve as feedback inhibitors for further enzyme activity. Sometimes enzyme activity is regulated by competitive binding at the enzyme active site. Often a feedback inhibitor will be the product of some metabolic pathway initiated by the enzyme being inhibited.
Allosteric enzyme regulation occurs when binding of a signal molecule at an allosteric site changes the shape of an enzyme. Binding by allosteric inhibitors prevents enzyme-substrate interaction. Allosteric activators bind to allosteric sites to change protein shape and they produce the key-in-lock enzyme substrate fit. In allosteric activation, the enzyme will not function until its shape is changed by the signal molecule. Allosteric enzyme regulation is also a part of many complex metabolic pathways.
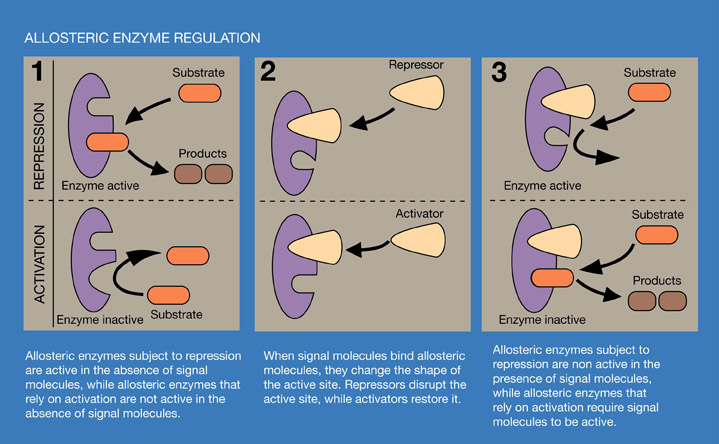
Diagram of an allosteric enzymatic reaction
Why is pH important to protein function?
The correct answer is A. Protein function depends on shape. Changes in pH mean changes in H+ concentration, which impacts protein function by disrupting H bonds.