username@email.com
username@email.com
Now we will cover the rates of reactions and what affects them, namely, temperature, pressure, surface area, catalysts, and various characteristics of the reactants in the system.
In the previous lesson, we reviewed the concepts of the ideal gas, the gas law, and molecular kinetics.
The very nature of chemistry is concerned with how substances change over time. This change over time is an aspect of kinetics called the reaction rate. As the masses of reactants in a given reaction decrease, the masses of products increase. For a reaction like dynamite exploding, this may seem like a trivial exercise in quantifying a process, but most reactions are not as dramatic or complete as dynamite exploding. Many reactions in the human body are maintained in a steady state within an equilibrium by virtue of these factors. Blood sugar metabolism, controlled by insulin, is one such example.
Reaction rates depend on many factors:
Reaction rates decrease as the concentration of the reactants decreases. If there are fewer particles to interact, the chance that those particles may come in contact diminishes. As the product particles accumulate, they also get in between potential reacting particles and this also decreases the rate of the reaction.
Generally, as the temperature increases, the increase in resulting particle motion increases the chances for particles to collide and interact. An increase in temperature usually increases reaction rates. An exception to this is with gases. Sometimes the increase in temperature will create greater distances between particles, unless there is a constant or increasing pressure to keep the distances between particles constant.
The greater the surface area of a reactant, the more easily it will have access to the other reactant. For example, powdered solids react more readily than large chunks of a solid. Many reactions proceed more quickly in water because the ions are separated and available for interaction, whereas in the solid dry form, they are ionically bonded together. In general, the smaller the particle size and more access it has to the other reactant, the greater the potential for interaction.
Some reactions have mechanisms that require more than one reactant to be present for successive steps to take place. Or multiple successive steps may be required to completely change one reactant into one product. The rates of these reactions depend on the rate-limiting mechanism that is specific to the individual reaction. These cannot be predicted easily and require actual experimentation to reveal the individual mechanisms. These types of reactions are often encountered with molecular or organic compounds.
Many of these complex reactions can be sped up tremendously using catalysts. Most biochemical reactions that occur in living systems have a number of catalysts that tightly regulate the speed of reactions with feedback systems. These include cascades of reactions that are signaled at the surface of a cell and ultimately regulate gene expression, to allow the cell to adapt to changing conditions. One example is the reaction that occurs when you have fever that results in the production of many millions of rapidly dividing white blood cells that protect the body against infection.
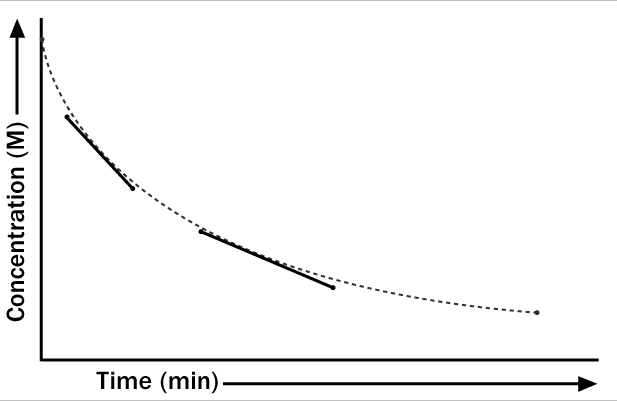
The average rate of reaction for a given period can be determined from the slope of the line on a graph in which concentration is plotted against time. The equation can be stated as:
Reaction Rate = K [A]n[B]m
K = Reaction Constant
A = Reactant & B = Reactant
n & m = Order Number (Superscript number)
You can see from this graph that the slope of the line changes as the availability of the reactants changes. The first solid line indicates a more rapid reaction than the second solid line in the graph.