 username@email.com
username@email.com
We are now going to review the four different kinds of reactions and how to balance a chemical equation. At the conclusion of this lesson, you should be able to identify and describe any reaction and effectively balance both sides of a chemical reaction.
We have reviewed the chemical naming rules and how to name any chemical, compound, or material based on a few important nomenclature protocols. This is a basic skill that will serve us well in the next lessons.
With our knowledge of atomic structure and, since elements are known to bond in regular, predictable patterns in whole number ratios, there are a number of quantitative relationships that can be determined using information from the periodic table.
A conveniently measured amount of any element that equals the mass in grams of that element is called a mole. One mole of hydrogen weighs 2 grams. One mole of chlorine weighs 70 grams. Just like a dozen equals twelve, a mole equals Avogadro’s number of atoms or 6.02 × 1023. 6.02 × 1023 molecules of hydrogen would weigh 2 grams. An equal number of chlorine molecules would weigh 70 grams. Chlorine is much heavier than hydrogen because it has more protons and neutrons. A similar analogy is that a dozen bowling balls would weigh more than a dozen golf balls. A mole is simply a very large number of atoms. You need a very large number of atoms to be able to measure something you can see, like a teaspoon of salt. The coefficient is given to determine the number of moles in the balanced equation. Using these mole ratios, you can substitute the grams for atomic mass units and calculate relative masses for reactants and products.
Here are some simple examples of the gram atomic weight for one mole of different common compounds.
Carbon dioxide: CO2
One mole (C) 12 grams + One mole (O2) 32 grams = 44 grams
Water: H2O
One-half mole (O2) 16 grams + One mole (H2) 2 grams = 18 grams
There are four basic types of chemical reactions. The first is the simplest and is known as synthesis, or a composition reaction. This type of reaction combines two or more substances to form at least one new compound. One example has already been given which is when sodium metal, a solid, combines with chlorine gas to form sodium chloride. The reaction can be symbolized in the following way:
Na (s) + Cl2 (g) → NaCl (s)
The reactants are on the left hand side of the arrow and the product(s) is (are) on the right. It can be read to mean that the reactants, sodium and chlorine, will react to form a single product, sodium chloride. In the chemical equation above, the (s) stands for solid, and the (g) stands for gas which indicates which state of matter the substance is in. Sometimes the arrow is read as “yields.” As you look at this equation, the natural question to ask is why are there three atoms on one side of the equation and only two atoms on the other? What happened to the extra chlorine atom? Good question. This equation is not a balanced equation, and matter doesn’t just disappear and appear. A more accurate way to correctly represent this reaction is as follows:
2Na (s) + Cl2 (g) a 2NaCl (s)
This can be understood by saying that two sodium atoms combine with one diatomic chlorine molecule to form two ionic units of sodium chloride.
Another aspect of chemical reactions is the physical properties of compounds, or how atoms and molecules fit together. If we look at the crystal structure of NaCl, we notice that it is a closely packed cube.
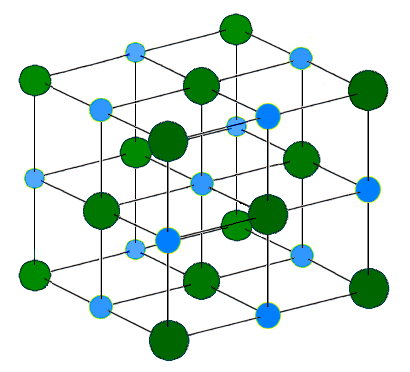
The sodium chloride crystal structure has each atom with six of its nearest neighbors in an octahedral geometric pattern. Na ions are in light blue and Cl ions are darker green for contrast.
All reactions are simply rearrangements of matter in more stable ways. New matter is never created, and no matter is ever destroyed. This is known as the law of conservation of matter; matter can neither be created nor destroyed, but matter simply changes form.
sodium + chlorine → sodium chloride
2 Na (s) + Cl2 (g) a 2 NaCl (s)
2 moles + 1 mole = 2 moles
2(23 amu) + 2(35 amu) = 2(58 amu)
46 amu + 70 amu = 116 amu
116 amu = 116 amu
Using the atomic weighs from the periodic table, you can see that two sodium atoms weigh 46 atomic mass units. One chlorine molecule weighs 70 atomic mass units. Two units of a sodium chloride crystal weigh 116 atomic mass units. The total mass of the reactants must equal the total mass of the product.
A second type of chemical reaction is known as a decomposition reaction. This is where a compound is broken down into two or more simpler parts. One example is the electrolysis of water. Water can be broken down in to its constituent elements, hydrogen and oxygen with the addition of electricity. The equation looks like this:
water → hydrogen + oxygen
2 H2O (l) → 2 H2 (g) + 2 O2(g)
2 moles → 2 moles + 1 mole
2(18 grams) = 2(2 grams) + 32 grams)
36 grams = 4 grams + 32 grams
Based on these quantitative relationships you can predict that 36 grams of water will yield 4 grams of hydrogen gas and 32 grams of oxygen gas. The lowercase (l) stands for liquid, which is what the state of water is in the reaction.
If carbon has a molecular weight of 12g and oxygen has a molecular weight of 16g, what does a mole of carbon dioxide weigh?
The correct answer is C, 44g. Since one mole carbon combines with two moles of oxygen to form two moles of carbon dioxide, the weight of a mole of this gas would be 12 + 16 + 16 = 44g.
A third type of reaction is called a single replacement reaction. This is where a more active element will replace a less active element in a compound. One example is when the metal zinc is dropped into a solution of hydrogen chloride to generate hydrogen gas. The equation would look like this:
zinc + hydrogen chloride → zinc chloride + hydrogen
Zn (s) + HCl (aq) → ZnCl2 (aq) + H2 (g)
The (aq) stands for aqueous, which means that the substance is dissolved in a water solution. Can you determine what coefficients are missing to make the equation a balanced one?
If you thought a 2 needed to be in front of the reactant HCl, you would be right. How many moles of zinc would be needed to react with 2 moles of hydrogen chloride to form 1 mole of aqueous zinc chloride?
You are correct if you thought the answer was one. One mole of zinc will react with 2 moles of aqueous hydrogen chloride to form one mole of zinc chloride and one mole of hydrogen gas.
If you started this reaction with 65 grams of zinc, how many grams of hydrogen would you expect to make?
Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
If your answer was 2 grams, you are correct!
Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
1 moles + 2 moles → 1 moles + 1 mole
1(65 grams) + 2(36.5 grams) = 1(136 grams)+ 1(2 grams)
138 grams = 138 grams
A fourth type of reaction is known as a double replacement reaction. These often occur when two soluble reactants are dissolved in water and one of the products is not soluble. These insoluble products drop out of solution as a precipitant and can make the solution appear cloudy. One example is when lead chloride and sodium sulfide are dissolved in water; a precipitant forms known as lead sulfide. This substance is believed to be the “snow” that precipitates on the surface of Venus. The reaction can be written as follows:
Lead chloride + sodium sulfide → lead sulfide + sodium chloride
PbCl2 (aq) + Na2S (aq) → PbS (s) + NaCl (aq)
Using the unbalanced equation above, determine what coefficients are missing to make the equation balanced.
The correct answer is C, you need a 2 to be in front of the product NaCl to make the equation balanced
How much will two moles of NaCl weigh in grams?
The correct answer is B, two moles of NaCl would weigh 116 grams. Since a mole of sodium = 23g and a mole of chloride = 35g, we just have to multiply these each by two and add them together (70 + 46 = 116g).
To summarize the four basic types of reactions:
Oxygen is a common component of the Earth’s atmosphere and therefore is readily available to react with most elements and compounds on Earth. With its relatively small size and high electronegativity, oxygen participates in a large number of chemical reactions. One special type of reaction that is a combination of two basic types of reactions (composition and single replacement) is called an oxidation reaction, first named because oxygen was the most common element to be observed in this type of reaction. Other electronegative elements are observed to react in the same manner.
Let’s take a common example of an oxidation reaction with a molecule of methane, CH4. Methane is also known as natural gas, or swamp gas. This gas is flammable because it reacts in the presence of a spark with oxygen and produces a flame. Natural gas stoves make use of this reaction for heating and cooking.
methane + oxygen → carbon dioxide + water
CH4 (g) + O2 → (g) → CO2 (g) + H2O (g)
Is this a balanced chemical equation? What coefficient(s) are needed to make this equation follow the law of conservation of mass?
Two moles of oxygen molecules and two moles of water are needed to balance this equation.
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
How many grams of carbon dioxide gas would be produced from reacting two moles of oxygen with methane gas?
The correct answer is 44 grams.
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
1 mole + 2 moles → 1 mole + 2 moles
16 grams + 2(32 grams) = 44 grams + 2(18 grams)
80 grams = 80 grams
Oxidation reactions are the basis for respiration in all living things because most organic molecules can be oxidized to produce energy. Proteins, lipids, and carbohydrates can all be used as fuels similar to the methane in this example. Oxidation reactions are essential for life on Earth to exist.
One very common type of a double replacement reaction occurs when an acid and a base react to form a salt and water. Examples are given below:
hydrochloric acid + sodium hydroxide → sodium chloride + water
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
sulfuric acid + magnesium hydroxide → magnesium sulfate + water
H2SO4 (aq) + Mg(OH)2 (aq) → MgSO4 (aq) + 2H2O (l)
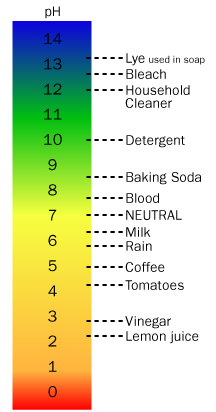
An equally strong acid and base will produce a neutral solution of salt water. The acidity of solutions can be of varying strengths and can be measured by what is called the pH. A solution will be labeled an acid if the pH is less than 7, a base when the pH is greater than 7, and neutral when the pH equals 7.
The pH is defined as the negative log of the hydrogen ion ([H+]) or hydronium ion ([H3O+]) concentration depending on what source you use. Remember that hydrogen ions are really just bare protons (positively charged nuclei) that are attracted to the lone pair of electrons in water molecules. The nature of this relationship determines the strength of the acid in water because some substances tend to give up more [H+] in water than others.