username@email.com
username@email.com
Now we are going to cover the chemistry of solutions, including some electrochemical concepts.
In the last lesson we took a quick look at oxidation and reduction reactions.
A solution is a combination of two or more pure substances equally dispersed throughout the solution. Using the common example of saltwater, the solvent is the water, the solute is the salt and the saltwater combination is the solution. If the solution has dissolved all the salt it can dissolve, it is called a saturated solution. If more salt can be dissolved, it is called an unsaturated solution. Sometimes, a solution can hold more than a saturated solution but a change in temperature or a seed crystal can precipitate the solution. These unstable solutions are called supersaturated. Only under certain changes such as a gradual cooling with no vibrations can a supersaturated solution be achieved.
Sometimes solution processes can be endothermic or exothermic. If the attraction of the solute for the solvent releases energy, it can become highly exothermic. An example of this would be dissolving an acid in water. These solutions get very hot. If the attraction of a solute for a solvent requires energy, such as ammonium nitrate in water, the temperature of the solution decreases and is endothermic. Instant heat packs and cooling packs use this principle to achieve the desired temperature simply by mixing an aqueous salt in water.
Water is considered to be the universal solvent, but it is chemically a polar solvent. This means that the molecules have the electrons shifted slightly to one side in the bond between the hydrogen and oxygen. Because this shifts the electron density to one side, a polar molecule is more positive on one side and less positive or more negative on the other side. Its polarity allows water to dissolve ionic substances that have positive and negative ions, such as sodium chloride. The positive part of the water molecule is attracted to the chloride ions and the negative part of the water molecule is attracted to the sodium ions. In terms of solutions, the phrase “like dissolves like” holds true. Water can also dissolve other polar substances, such as ethanol, C2H5OH.
Examples of nonpolar solvents are gasoline, kerosene, oils, and waxes. These substances don’t dissolve in water. However, they dissolve in each other. Crude oil is really just a naturally found, complex mixture of hydrocarbons from which methane, propane, butane, and octane are simply dissolved components.
How do you know how much solute is dissolved in a solution? One crude way is to examine the density. As more particles dissolve in a solution, the solution becomes denser. That is why ships sail higher on saltwater than freshwater (and it is easier to float in the ocean than in the swimming pool). More precise ways to measure the concentration are as follows:
Some properties of solutions don’t depend on the substances dissolved but are simply a result of the number of separate particles in the solution. These are called colligative properties.
One of these properties is freezing point depression. If one dissolves a number of particles in a solution, this interferes with the ability of the water molecules to line up properly as the temperature drops and particle motion slows down. Because the particles are in between the water molecules the temperature must be much lower to turn the liquid into a solid. This is the principle behind the salt placed on icy roads.
Another property is boiling point elevation. A saltwater solution boils at a higher temperature than a pure water solution. This is because the salt particles interfere with the water molecules.
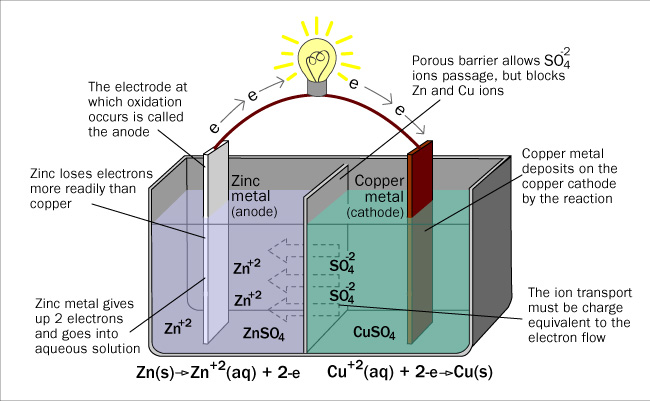
As described earlier, electrochemical cells (batteries) are applications of oxidation and reduction, or redox reactions. As electrons flow from less electronegative elements to more electronegative elements, via ionic solutions, a current can be achieved. Look at the diagram below. Electrons travel from the zinc to the copper plate. These extra electrons supply the copper sulfate ions of copper with the electrons to become elemental copper and plate, onto the copper cathode. The sulfate ions travel to the anode to pick up the zinc atoms that have lost two electrons to become zinc ions. This supports the electron flow through the cell. This flow has the ability to do work such as light up a light bulb or produce heat.