 username@email.com
username@email.com
In this lesson, we will explore the composition of our atmosphere. We will also take a look at the role water and heat energy play in climate and weather.
The geologic time scale developed as a relative scale based upon observations of rock strata.
The age of terrestrial rock strata has been determined by radioactive dating methods.
These methods use naturally radioactive isotopes with known half-lives to determine the age of rocks.
The age of the Earth is 4.5 billion years old based upon measuring the age of extraterrestrial sources such as meteorites and moon rocks that formed at the same time as the Earth.
Our atmosphere is a delicate life-giving blanket of air. This air surrounds us all of the time. It goes from the ground at our feet to hundreds of miles over our heads. We are confined to our environment of air just like fish are confined to their environment of water. We can feel air as we move through it or as it moves around us so we know that it is a physical material. Air is a tasteless, odorless, and for the most part, invisible gas.
Air is a mixture of gases. The table below lists the major gaseous components of our atmosphere.
| Gas | Symbol | Volume |
|---|---|---|
| Nitrogen | N2 | 78.084% |
| Oxygen | O2 | 20.947% |
| Argon | Ar | 0.934% |
| Carbon Dioxide | CO2 | 0.033% |
| Neon | Ne | 18.2 parts per million |
| Helium | He | 5.2 parts per million |
| Krypton | Kr | 1.1 parts per million |
| Sulfur dioxide | SO2 | 1.0 parts per million |
| Methane | CH4 | 2.0 parts per million |
| Hydrogen | H2 | 0.5 parts per million |
| Nitrous Oxide | N2O | 0.5 parts per million |
| Xenon | Xe | 0.09 parts per million |
| Ozone | O3 | 0.07 parts per million |
| Nitrogen dioxide | NO2 | 0.02 parts per million |
| Iodine | I2 | 0.01 parts per million |
| Carbon monoxide | CO | trace |
| Ammonia | NH3 | trace |
You probably noticed that the majority of the gas in our atmosphere is nitrogen. In fact, the gases nitrogen, oxygen, argon, and carbon dioxide combine to make up 99.998% of the volume of the atmosphere.
Carbon dioxide and water are greenhouse gases. These gases get this classification because of their ability to trap heat in the atmosphere. Sunlight traveling through the atmosphere is absorbed by the Earth’s surface. It is then re-emitted as infrared radiation into the lower atmosphere where it is absorbed by carbon dioxide and water vapor. This infrared radiation is re-emitted and absorbed many times over by carbon dioxide and water, which warms the lower atmosphere.
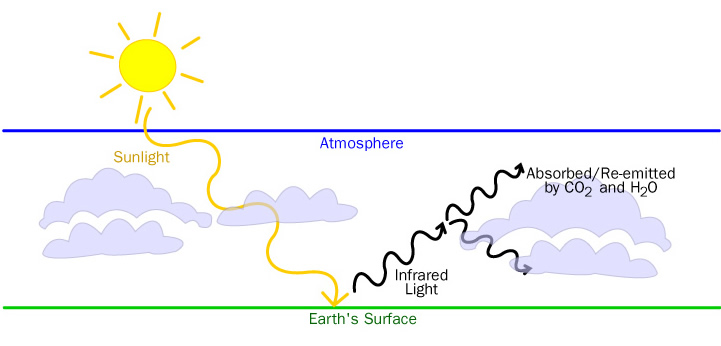
Increased atmospheric carbon dioxide is a concern because global temperatures tend to increase as levels of ‘green house’ gases increase. Ocean water absorption and photosynthesis will continue to be major processes that help to moderate the concentration of atmospheric carbon dioxide.
Water vapor is a small but climatologically significant component of our atmosphere. Contained mostly in the lower atmosphere, water vapor concentrations vary widely depending upon geography and climate. In a desert region, the concentration of atmospheric water vapor could be almost as low as 0% most of the time. The same thing can be said about the climate at the poles. The air in these cold regions is very dry with extremely low concentrations of water vapor. The maximum amount of atmospheric water vapor that can be found in any climate is 4%. Areas with this high concentration of water vapor are typically tropical areas.
Weather phenomena such as clouds, precipitation, thunderstorms, and hurricanes are produced by the exchange of heat energy in the atmosphere through the condensation and evaporation of water. These same processes are responsible for transporting liquid water from the earth’s surface into the atmosphere and back again through the water cycle.
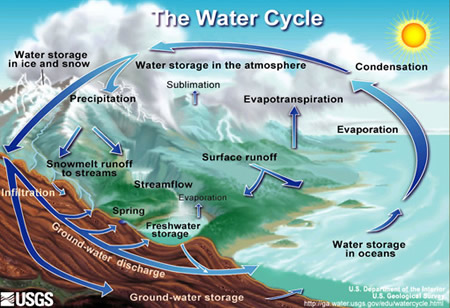
The water cycle not only circulates water through the atmosphere, but it also does the same thing with energy in the form of heat. To illustrate this, let’s follow one kilogram of water into the atmosphere. Each kilogram of liquid water that absorbs sunlight gains 2260 kilojoules of energy to make the transition to water vapor. This water vapor would probably stay close to the surface if it were not for convection in the atmosphere. Convection currents carry water vapor to higher altitudes.
As our kilogram of water vapor gains altitude, it expands and cools. This process is referred to as an adiabatic process since the cooling that occurs is a result of the expansion and subsequent drop in pressure due to the gain in altitude. The rate at which it expands and cools depends upon the conditions present in the atmosphere at that time. When this kilogram of water vapor cools down to the dew point, it begins to condense on dust particles in the atmosphere forming clouds. The act of condensation releases 2260 kilojoules of energy into the surrounding atmosphere. If the one-kilogram sample cools enough to form ice crystals, it can release an additional 2842 kilojoules of heat energy into the atmosphere. This heat energy is what drives the various forms of weather phenomena we experience.