 username@email.com
username@email.com
Energy must be added to matter in order to increase its temperature. The amount of energy (Q) needed to heat any material depends on three quantities:
The equation looks like this:
Q = cmΔT
The specific heat capacity of a material can be determined using an instrument called a calorimeter. To use a calorimeter, begin by heating a sample of the material to a known temperature (like the boiling point of water). Then, place the sample in an insulated container that is filled with a known mass of water at a known temperature. Eventually, both the water and the sample reach a common temperature (equilibrium). Since the system is isolated in an insulated container, heat energy from the sample transfers to the water and the container. According to the law of conservation of energy, the heat energy lost by the sample equals the heat energy gained by the water and the container. Using this law along with the equation for heat above, the specific heat capacity of the material may be calculated.
In the chemistry review, you studied the following three main points for the kinetic theory of gases:
The molecules in real gases, however, have complex shapes that are capable of having electromagnetic interactions. At high pressures and at low temperatures these interactions may be significant. At most conditions, however, the ideal gas model may be adequate to describe how gases interact. Molecules in ideal gases are sufficiently far apart so they have very few interactions. In fact, we can assume that the interactions they do have are elastic, meaning that they occur without any loss in kinetic energy. Although no gas is truly “ideal,” the ideal gas model is appropriate in a large number of cases.
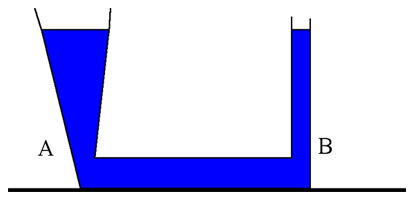
For example, the pressure on top of the water shown above is 1.01 · 105 Pa and the density of water is 1.000 · 103 kg/m3. Both points A & B are 2.0 meters below the water level. How do the pressures at points A & B compare? Try using the equation above to calculate each pressure.
Gas pressure is the result of the collision of gas particles with an object. What would happen to the pressure if the number of particles in a certain volume were increased? Kinetic theory of ideal gases predicts the there will be more collisions between the particles and thus more pressure. Besides depending on the number of particles, pressure also depends on the average kinetic energy of the particles, or temperature.
What if different gases are combined at a constant volume and temperature? At constant temperature, the average kinetic energy of the mixture is constant and the type of gas particle is not important. Each gas will contribute to the new pressure as it is combined with the mixture. The contribution of each gas in the mixture makes to the total pressure is called the partial pressure. John Dalton (1766-1844) proposed that in a mixture of gases, the total pressure is simply the sum of all the partial pressures in the gases. This is called the law of partial pressures.
For example, consider two gas containers. One is filled with oxygen gas at a pressure of 300 kPa, another is filled with nitrogen gas at a pressure of 400 kPa, and both containers are at the same temperature of 25 ° C. If both gases are combined in the same container, what is the pressure of the combined gases?
According to the law of pressures we add the pressure of the oxygen (P 1) to the pressure of the nitrogen (P 2) as follows:
P total = P 1 + P 2 = 400kPa + 300kPa = 700kPa
Heat is a “special form” of energy, right? Well, not really. Energy is energy. As you’ve already learned, energy cannot be created or destroyed, but it may be transferred, transformed, and/or stored. The first law of thermodynamics is another name for the law of conservation of energy for a system. If heat flows into a system from its surroundings, the system gains internal energy as the random kinetic energy of its particles increases. Likewise, a system will also gain energy if an outside agent does work on the system through the application of forces.
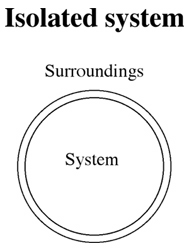
The figure below shows an isolated (insulated) system in which the energy stays constant.
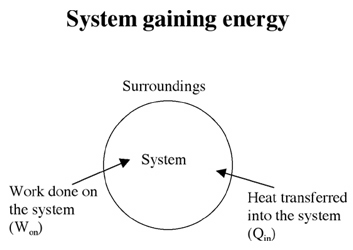
ΔE int = Q in + W on
where Q in is the heat that transfers into the system and W on is the work done on the system.
If the system is doing work on its surroundings, the internal energy of the system decreases. Then W on is considered to be negative (see figure below). If heat flows out of the system, the internal energy of the system also decreases and Q in is considered to be negative.
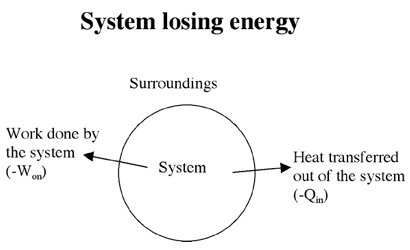
For example, let’s say that 5 joules of work are done by stirring a beaker of warm water. During this time, 15 joules of heat leave the poorly insulated container. By how much did the internal energy of the container/water system change?
Assuming that the beaker is the system, the process of stirring added heat energy to the system making W on = + 5 J. Since heat flowed out of the container, the system loses energy making Q in = -15 J. Therefore,
ΔE int = Q in + W on
ΔE int = -15J + 5J
ΔE int = -10J
The system lost 10 joules of internal energy.
Ideal gases may do work through changes in pressure, volume, and/or temperature. Thermodynamic processes of ideal gases are represented on a pressure-volume (p-V) diagram. By knowing both the pressure and volume at any point on the graph, the state of the gas is completely specified. Three common thermal processes for enclosed gases are isobaric, isochoric, and isothermal.
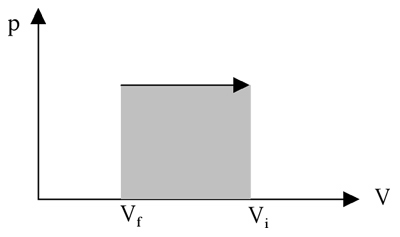
An isobaric process is one that occurs at constant pressure. For example, a heated gas in a container expands to lift a piston that applies a constant pressure. In the process of lifting the piston, work is done by the gas in increasing its volume. This is represented as follows on a p-V diagram:
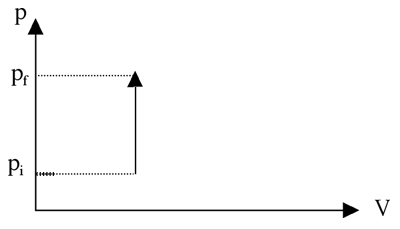
An isochoric process is one that occurs at constant volume. For example, a heated gas in a closed container has no room to expand; therefore its pressure must rise. Even though the gas exerts more and more force on the walls, the walls do not move and the gas does no work on the container. This is process is represented on the following p-V diagram:
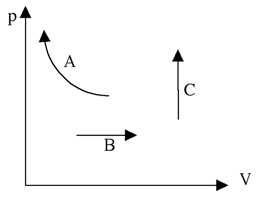
1. Which line demonstrates an ideal gas heated isochorically?
2. Which line demonstrates isothermal compression of an ideal gas?
3. Which line demonstrates isobaric expansion of an ideal gas?
Refrigerators and air conditioners are devices that rely on the laws of thermodynamics in order to operate. Both devices move heat energy from one location to another through the change of phase using a refrigeration fluid (like Freon) Actually, they are heat engines that, instead of using the P-V concept to do work on their surroundings, remove heat energy (the ability to do work) from an enclosed space and expel that energy as a waste product thereby producing a cooling effect on the enclosed space. Stand outside near a working air conditioner and you can feel the expelled heat.
The second law of thermodynamics states that heat naturally flows from warm regions to cold regions. This is bad news for refrigerators. In order to keep the interior of a refrigerator cold, a pump must do work to force the refrigerant through small openings inside the cooling unit. As the refrigerant evaporates, unwanted interior heat is transferred into it. Thus, the refrigerant enters the exterior condenser coils of the refrigerator as a gas. When the gas condenses back into the liquid phase in these coils, it releases heat energy into the kitchen (the surroundings) before it is pumped back into the cooling unit to repeat the cycle. Air conditioners operate in the same way as refrigerators, except they pump heat energy from inside the house to the outdoors.
Let’s check your understanding with the following question. Is it possible to cool your house by leaving the door of the refrigerator open?
While you initially feel cooler when the door of the refrigerator is open, the refrigerator will start to do more work to move the heat from inside the refrigerator to the outside. Unfortunately, that heat will enter right back into the kitchen from the coils. In addition, according to the second law of thermodynamics, energy from the work is also deposited in the kitchen.